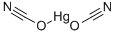Chemical Properties
Gray, crystalline powder.Soluble in alcohol, ammonium hydroxide, and hot water; slightly soluble in cold water.
Uses
Manufacture of caps and detonators for producing explosions of military, industrial, and sporting
purposes.
Uses
Mercury fulminate [Hg(CNO)2] is very explosive and is used to manufacture blasting caps
and detonators.
Uses
Mercury fulminate is used as a primaryexplosive to initiate boosters.
General Description
A slurry or wet mass of white crystals. Contains at least 20% water or water-ethyl alcohol mixture. May explode from shock, heat, flame, or friction when dry. May explode under prolonged exposure to heat. Primary hazard is blast of an instantaneous explosion, not flying projectiles or fragments. A widely used initiating detonator for high explosives.
Reactivity Profile
MERCURY FULMINATE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper . Contact with sulfuric acid causes explosion, [Urbanski, 1967, vol. 3, 135, 149]. Aqueous ammonia and Hg react to form an explosive solid, likely a fulminate. (Thodos, G. Amer. Inst. Chen. Engrs. J., 1964, 10, 274.).
Hazard
Explodes readily when dry, keep moist till
use, an initiating explosive. Highly toxic.
Health Hazard
Fire may produce irritating, corrosive and/or toxic gases.
Health Hazard
Mercury fulminate is a highly toxic compound,exhibiting the toxicity symptoms ofmercury. No toxicity data are available onthis compound.
Fire Hazard
MAY EXPLODE AND THROW FRAGMENTS 1600 meters (1 MILE) OR MORE IF FIRE REACHES CARGO.
Safety Profile
An explosive sensitive
to flame, heat, impact, friction, intense
radiation, or contact with sulfuric acid. Selfexplodes. Dangerously flammable; should be
kept moist until used. Incompatible with
sulfuric acid. When heated to decomposition
it emits very toxic fumes of Hg and NOx. See also MERCURY COMPOUNDS and
FULMINATES.