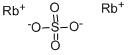Preparation
Rubidium sulfate can be prepared by neutralization of a solution of rubidium hydroxide or carbonate with sulfuric acid:
2RbOH + H2SO4 → Rb2SO4 + 2H2O
Rb2CO3 + H2SO4 →Rb2SO4 + H2O + CO2
Alternatively, Rb sulfate may be obtained by treating a hot solution of rubidium aluminum sulfate (rubidium alum) with ammonia solution.
Aluminum hydroxide precipitates. The product mixture is filtered. The filtrate on evaporation crystallizes rubidium sulfate.
Description
Rubidium sulfate is a White orthogonal crystal. The molecular formula of the compound is Rb
2SO
4 and the molecular weight of rubidium sulfate is 266.999 g/mol. Rubidium Sulfate is a moderately water and acid soluble. Rubidium source for uses compatible with sulfates. Sulfate compounds are salts or esters of sulfuric acid formed by replacing one or both of the hydrogens with a metal.
Chemical Properties
colorless crystal(s) [MER06] [HAW93]
Physical properties
White orthogonal crystal; density 3.6 g/cm
3; melts at 1,050°C; very soluble in water, 36 g/100g at 0°C and 82 g/100g at 100°C.
Uses
Rubidium sulfate can be used for making other rubidium salts as the raw materials, for water treatment, or for hematology stain. It is also usedd on a ceramic bead and used as thermoionic detector. Rubidium sulfate is also used as a cathartic.
Purification Methods
Crystallise the sulfate from water (1.2mL/g) between 100o and 0o.