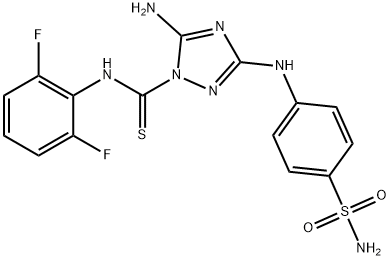
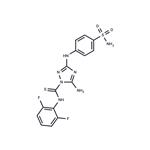
Description
Cyclin-dependent kinases (Cdks) are key regulators of cell cycle progression and are therefore promising targets for cancer therapy. Cdk1/2 Inhibitor III is a cell-permeable inhibitor of Cdk1/cyclin B and Cdk2/cyclin A (IC
50s = 0.6 and 0.5 nM, respectively). It less potently inhibits CDC2-like kinases 1 and 3, VEGFR2, and GSK-3β (IC
50s = 8.9, 29, 32, and 140 nM, respectively) and is without effect against a panel of other kinases. Cdk1/2 Inhibitor III blocks the growth of several cancer cell lines (IC
50 values range from 20 to 92 nM).
Uses
Cdk1/2 Inhibitor III is a cyclin-?dependent kinase (CDK) inhibitor. Cdk1 and Cdk2 performs inhibitory activities, and in vitro cellular proliferation in various human tumor cells.
in vitro
cdk1/2 inhibitor iii was identified as a cell-permeable inhibitor of cdk1/cyclin b and cdk2/cyclin a and could less potently inhibit cdc2-like kinases 1 and 3, vegfr2, and gsk-3β. cdk1/2 inhibitor iii was found to be lack of effect against a panel of other kinases. moreover, cdk1/2 inhibitor iii could block the growth of various cancer cell lines (ic50 values range from 20 to 92 nm) [1].
in vivo
the in-vivo efficacy of compound 3b, a structurally close cdk1/2 inhibitor iii analog, was examined in the a375 human melanoma cell xenograft model. doses at 125, 100, and 75 mg/kg were administered once a day for 32 days and tumor size was measured every 4 days. the results showed that in the 125 mg/kg group, there was one nontreatment-related death but the remaining four animals experienced stable disease. in addition, compound 3b administered at 100 and 75 mg/kg led to mean day of survival values of 50.1 and 48.5 days, respectively, with only one treatment-related death in the 100 mg/kg group [1].
IC 50
0.6 and 0.5 nm forcdk1/cyclin b and cdk2/cyclin a, respectively
References
[1] lin, r. ,connolly, p.j.,huang, s., et al. 1-acyl-1h-[1,2,4]triazole-3,5-diamine analogues as novel and potent anticancer cyclin-dependent kinase inhibitors: synthesis and evaluation of biological activities. journal of medicinal chemistry 48(13), 4208-4211 (2005).