Chemical Properties
white to almost white crystalline powder
Uses
Metal-chelating agent
Uses
As a Penicillin metabolite, L-Penicillamine can be used in the treatment of Wilson’s disease, Cystinuria, Scleroderma and arsenic poisoning.
Uses
L-Penicillamine is a metabolite of penicillin. L-Penicillamine is used in the treatment of Wilson’s disease, Cystinuria, Scleroderma and arsenic poisoning.
Indications
Penicillamine (Cuprimine) can be used to treat acute,
severe rheumatoid arthritis, producing reductions in
joint pain, edema, and stiffness.The response to penicillamine
is usually delayed (4–12 weeks), and remissions
can last several months after withdrawal of treatment.
Radiographic evidence of this drug’s efficacy is limited;
thus, penicillamine is seldom used to treat rheumatoid
arthritis.
Definition
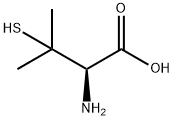
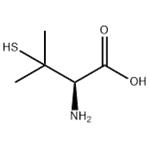
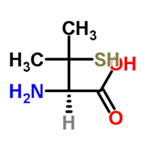
ChEBI: The L-enantiomer of penicillamine.
reaction suitability
reaction type: solution phase peptide synthesis
Mechanism of action
The mechanism of action of penicillamine is
unknown, but some evidence suggests that it may involve
the inhibition of angiogenesis, synovial fibroblast
proliferation, or transcriptional activation. Because
penicillamine can chelate copper and promote its excretion,
it is used to treat Wilson’s disease (hepatolenticular
degeneration) and has also been used in mercury
and lead intoxication.
Pharmacology
Penicillamine is readily absorbed from the GI tract
and is rapidly excreted in the urine, largely as the intact
molecule. Gradually increasing its dose minimizes side
effects, which necessitate discontinuance of penicillamine
therapy in perhaps one-third of patients. The
most common side effects are maculopapular pruritic
dermatitis, GI upset, loss of taste sensation, mild to occasionally
severe thrombocytopenia and leukopenia,and mild proteinuria, which at times may progress to
the nephritic syndrome. Discontinuance of therapy usually
results in a rapid disappearance of side effects.
Safety Profile
A poison by intraperitoneal route. Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx and SOx.
Purification Methods
Same as preceding entry for its enantiomer. [Beilstein 4 IV 3228.]