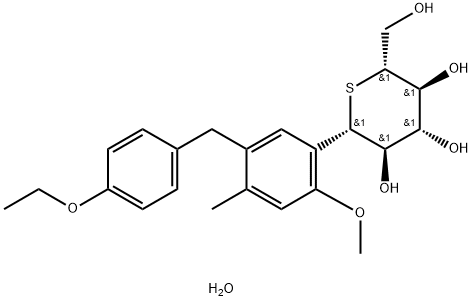
Description
Luseogliflozin hydrate, which is an SGLT2 inhibitor approved in
Japan in March 2014 for the treatment of type 2 diabetes, was discovered
by Taisho Pharmaceutical and jointly developed and marketed
with Novartis as Lusefi®. Luseogliflozin selectively binds
and inhibits human SGLT2 with a Ki of 1.10 nM and IC50 value of
2.26 nM.
Synthesis
The most likely synthetic route capable of producing luseogliflozin
on process scale has been reported by Taisho. Commercially available 4-methoxy-
2-methyl-benzoic acid (160) was brominated in the presence
of a catalytic amount of iron powder, generating a 1:1 mixture of
3- and 5-bromo derivatives which were separable by recrystallization
from methanol to provide the desired regioisomer 161 in 34%
yield. Next, benzoic acid 161 was treated with oxalyl chloride to
give the corresponding acyl chloride 162, which underwent a Friedel¨C
Crafts reaction with ethoxybenzene (163) to afford 164 in 82%
yield for the two step sequence. The dibenzylic ketone 164 was
reduced using triethylsilane and boron trifluoride to give aglycon
165 in 99% yield. The Grignard reagent prepared from bromide
165 was then alkylated with thiolactone 166 (prepared from 5-
thio-D-glucose penta-O-acetate (169) as illustrated in Scheme 27)
to give rise to hemithioacetal 167 in 75% yield. This compound
underwent stereoselective reduction to provide thioglycoside 168
in 77% yield. Hydrogenation of 168 resulted in global debenzylation
to provide the desired product luseogliflozin (XIX) in 81%
yield.
The preparation of key thiolactone 166 started from 5-thio-Dglucose
penta-O-acetate (169) ; 169
could be prepared by eight steps from commercially available Dglucurono-
3,6-lactone. The anomeric acetyl group of 169
was selectively removed by hydrazine and acetic acid at room temperature
giving compound 170 in 70% yield. Subsequently, the
anomeric hydroxyl group of 170 was protected with tetrahydropyran.
Then, all acetyl groups of 171 were removed by Zempl¨|n
deacetylation, and the resulting hydroxyl groups of 172 were further
protected by benzyl groups using benzylbromide with sodium
hydride to generate 173. The tetrahydropyranyl group of 173 was
removed using pyridinium p-toluenesulfonate (PPTS), followed by
DMSO oxidation to provide 166 in 82% yield, by way of 174.