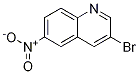
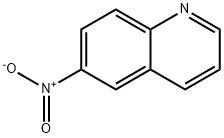
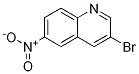
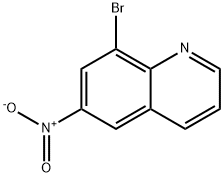
Synthesis
General procedure for the synthesis of 3-bromo-6-nitroquinoline and 8-bromo-6-nitroquinoline from 6-nitroquinoline: a mixed solution of 6-nitroquinoline (28.1 g, 161 mmol) and N-bromosuccinimide (28.7 g, 161 mmol) in acetic acid (280 mL) was heated and reacted for 17 hours at 50 °C. Upon completion of the reaction, the precipitated solid was collected by filtration and washed sequentially with ether, water and then ether to give 14.7 g (27% yield) of Intermediate 2 (93% purity). The organic phase was concentrated to dryness and the residue was purified by silica gel column chromatography using a mobile phase gradient (from 50% petroleum ether/50% dichloromethane to 100% dichloromethane). The pure grades were collected and the solvent concentrated to give 2.25 g (4% yield) of intermediate 2 and 16.6 g of residue. The residue was purified by a second silica gel column chromatography with a mobile phase of 50% petroleum ether/9:1:0.2 cyclohexane/ethyl ether/dichloromethane. The pure grades were collected and the solvent was concentrated to give 14.1 g (28% yield) of Intermediate 1.