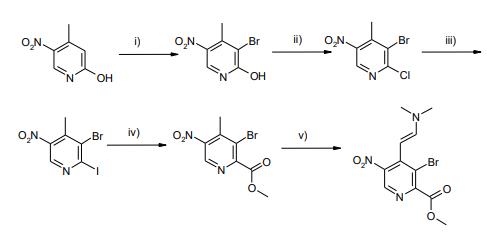
Preparation
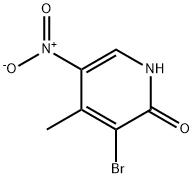
3-bromo-4-methyl-5-nitropyridin-2-ol synthesis: 4-Methyl-5-nitropyridin-2-ol (104.5 g, 0.68 mol) was suspended in AcOH (1000 mL) and bromine (208 mL, 6 eq) was added dropwise in 1.5 h. The mixture was stirred for 5 min, the poured into icewater (2000 mL) and stirred for 1 h. The resulting suspension was filtered and the residue was washed with water (3*100 mL), dried in vacuo and then stripped with toluene (2*500 mL) and CH3CN (500 mL). This afforded 3-bromo-4-methyl-5-nitropyridin-2-ol as a yellow solid (143.8 g, 91%).