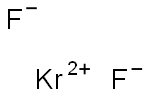Chemical Properties
colorless solid; decomposes rapidly at room temp; tetr, a=0.6533 nm, c=0.5831 nm; vapor pressure at 0°C is 29mm Hg; thermodynamically unstable, can be stored at ?78°C; prepared by electrical discharge of Kr and F2 at ?183°C; used in certain types of electric light bulbs [MER06] [KIR78] [COT88]
Physical properties
White tetragonal crystal; sublimes under vacuum at 0°C; decomposes slowly at 25°C; density 3.24 g/cm
3; reacts with water; vapor pressure 30 torr at 0°C; slightly soluble in liquid fluorine.
Uses
As gas filler in special purpose electric bulbs. To initiate and sustain the arc in metal-vapor discharge tubes, such as mercury and sodium vapor lamps. To determine surface areas of fine solids by adsorption techniques.
Preparation
Krypton difluoride may be prepared by the reaction of krypton with fluorine in an electric discharge at low pressure and liquid oxygen temperature. Also it may be made by irradiating krypton with ultraviolet rays in a fluorine—argon gas mixture at liquid helium temperature (-196°F).
Kr + F
2 → KrF
2 The product can be stored at -78°C without decomposition
.