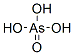Description
Arsenic acid, H3Aso4.½H20, white translucent crystals, soluble in water and alcohol; used in insecticides, glass making, and defoliants. Also known as orthoarsenic acid.
Description
Arsenic acid has the molecular formula H3AsO4.
More descriptively written as AsO(OH)3, this colorless
acid is the arsenic analog of phosphoric acid. Arsenate
and phosphate salts behave very similarly. Arsenic
acid as such has not been isolated, but only found in
solution where it is largely ionized. Its hemihydrate
form (H3AsO4·1/2H2O) does form stable crystals. Crystalline
samples dehydrate with condensation at 100 °C.
Arsenic acid is a tetrahedral species of idealized
symmetry C3v with As—O bonds lengths ranging from
1.66 to 1.71? . Being a triprotic acid, its acidity is
described by three equilibria:
H3AsO4 ? H2AsO4
- + H
+ (pk1 =10
-2.19)
H2AsO4 ? 5HasO4
2- + H
+ (pk2 = 10
-6.94)
HasO4 2 ? 5AsO4
3- + H
+ (pk3 = 10
-11.5)
Preparation
Arsenic acid is prepared by treating arsenic trioxide
with concentrated nitric acid or by combination of
arsenic acid with water. The latter reaction is very
slow. It is also formed when metaarsenic or pyroarsenic acid is treated with cold water.
Potential Exposure
It is used as a wood treatment, dryingagent, soil sterilant, and to make other arsenates. It has beenused as a cotton defoliant.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.Note to physician: For severe poisoning BAL [BritishAnti-Lewisite, Dimercaprol, dithiopropanol (C3H8OS2)]has been used to treat toxic symptoms of certain heavymetals poisoning—including arsenic. Although BAL isreported to have a large margin of safety, caution must beexercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with1-ephedrine sulfate (CAS: 134-72-5). For milder poisoningpenicillamine (not penicillin) has been used, both withmixed success. Side effects occur with such treatment andit is never a substitute for controlling exposure. It can onlybe done under strict medical care.
storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Arsenic acid must be stored to avoid contact with heat andchemically active metals (such as potassium, sodium, magnesium, and zinc) since violent reactions occur. Store intightly closed containers in a cool, well-ventilated area awayfrom heat. A regulated, marked area should be establishedwhere this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045.
Shipping
Arsenic acid requires a shipping label of“POISONOUS/TOXIC MATERIALS.” The Hazard Class is6.1 and the Shipping Group is II for solid and I for the liquidform.[19, 20]
Incompatibilities
Incompatible with sulfuric acid, caustics,ammonia, amines, isocyanates, alkylene oxides, oxidizers,epichlorohydrin, vinyl acetate, amides. Avoid contact withchemically active metals. Corrodes brass, mild steel, andgalvanized steel. Contact with acids or acid mists releasesdeadly arsine gas.
Short Term Exposure
Skin contact can cause irritation,itching, burning sensation, and rash. Eye contact can causeirritation and burns. Inhalation can cause irritation of therespiratory tract. High exposure can cause poor appetite,nausea, vomiting, and muscle cramps. High exposure cancause nerve damage with numbness, “pins and needles” sensation, weakness of the arms and legs. The oral LD50 for ratis 48 mg/kg. Ingestion of 130 mg of arsenic may be fatalto humans. Smaller doses may become fatal since arsenicaccumulates in the body.
Long Term Exposure
Arsenic acid is a mutagen that maycause changes to genetic material and an animal teratogen.Can cause an ulcer of the “bone” dividing the inner nose. Itcan cause disturbed sleep, thickening of the skin with patchareas of darkening and loss of pigment, or the developmentof white lines in the nails.
Personal Protective Methods
Where possible, encloseoperations and use local exhaust ventilation at the site ofchemical release. If local exhaust ventilation or enclosureis not used, respirators should be worn. A regulated,marked area should be established where arsenic acid ishandled, used, or stored. Wear protective gloves and clothing to prevent any reasonable probability of skin contact.Safety equipment suppliers/manufacturers can providerecommendations on the most protective glove/clothingmaterial for your operation. All protective clothing (suits,gloves, footwear, headgear) should be clean, available eachday, and put on before work. Contact lenses should not beworn when working with this chemical. Wear full facepiece respiratory. Employees should wash immediatelywith soap when skin is wet or contaminated. Provide emergency showers and eyewash. Specific engineering controlsare required under OSHA 1910.1018, Inorganic Arsenic.See also NIOSH Criteria Document #75-149, “InorganicArsenic.”
Fire Extinguishing
Extinguish fire using an agentsuitable for type of surrounding fire. Arsenic acid itself doesnot burn. Poisonous gases are produced in fire, includingarsine and oxides of arsenic. If material or contaminatedrunoff enters waterways, notify downstream users of potentially contaminated waters. Notify local health and fire officials and pollution control agencies. From a secure,explosion-proof location, use water spray to cool exposedcontainers. If cooling streams are ineffective (venting soundincreases in volume and pitch, tank discolors, or shows anysigns of deforming), withdraw immediately to a secure position. If employees are expected to fight fires, they must betrained and equipped in OSHA 1910.156. The only respirators recommended for firefighting are self-containedbreathing apparatuses that have full face-pieces and areoperated in a pressure-demand or other positive-pressuremode.
Disposal Method Suggested
Dissolve in a minimum of concentrated hydrochloric acid. Dilute with water until whiteprecipitate forms. Add HCl to dissolve. Saturate with H2S;filter and wash precipitate and return to supplier.Alternatively, precipitate with heavy metals, such as lime orferric hydroxide in lieu of H2S. Consult with environmental regulatory agencies for guidance onacceptable disposal practices. Generators of waste containing this contaminant ( $ 100 kg/mo) must conform withEPA regulations governing storage, transportation, treatment, and waste disposal.