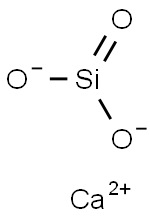Description
“Wollastonite” occurs as a calcium inosilicate
mineral that may contain small amounts of Fe, Mg and
Mn substituting for calcium.
The manufactured salt is
usually white. It forms within the earth when impure
limestone is subjected to high temperature and pressure,
sometimes in the presence of silica-bearing fluids as in
metamorphic rocks. It is named after the English
chemist and mineralogist William Wollaston (1766–
1828).
Wollastonite crystallizes as a triclinic crystal with
the space group, 1, and the lattice constants: a = 7.94? ,
b = 7.32 ? , c = 7.07 ? ; α= 90.03°, β = 95.37°, γ = 103.43°
and six formula units per cell.
Chemical Properties
White to brown, red, gray, yellow solid;
vitreous to pearly luster. Mohs hardness 4.5–5.
Physical properties
Wollastonite occurs as bladed crystal masses,
single crystals can show an acicular particle shape
and usually it exhibits a white color, but sometimes
cream, gray or very pale green. The melting point of
wollastonite is about 1540°C.
Uses
Ceramics; paint extender; welding rod coatings;
rubber filler; silica gels; paper coating; filler
in plastics, cements, and wallboard; mineral wool;
soil conditioner.
Uses
Wollastonite is used primarily in ceramics, friction products
(brakes and clutches), metalmaking, paint filler, and
plastics.
Definition
A natural calcium silicate found in metamorphic
rocks.
Production Methods
Wollastonite is a natural calcium silicate that typically occurs
in deposits with other silicate minerals. When crushed, it
tends to cleave into particles that have length to diameter
ratios of 7 or 8 to 1. Fibrous forms of wollastonite are not
uncommon.
General Description
White or slightly cream-colored powder. pH (aqueous slurry) 8.0 to 10.0.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
These substances undergo chemical reactions only under relatively severe circumstances or in the presence of an effective catalyst that promotes reaction. They are resistant to ignition, although they may become flammable at very high temperatures. They will be resistant to oxidation/reduction, except in the most severe conditions. These materials may be nontoxic.
Hazard
Questionable carcinogen
Fire Hazard
Flash point data for CALCIUM SILICATE are not available; however, CALCIUM SILICATE is probably nonflammable.
Safety
Calcium metasilicate is potentially harmful to humans. It can cause eye irritation and is harmful if swallowed. Prolonged exposure may result in symptoms such as chronic bronchitis, fibrosis of the lungs and pleural parenchyma.