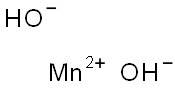Preparation
Manganese(II) hydroxide is obtained as a white precipitate by adding a solution of sodium or potassium hydroxide to a solution of manganese(II) salt, such as manganese(II) chloride:
Mn2+ + 2OH¯ → Mn(OH)2
The white precipitate rapidly turns brownish-pink in air. The reaction does not occur with ammonia in the presence of ammonium salt.
The hydroxide also is found in nature as mineral pyrochroite in the form of white transparent leaflets. The white leaflets turn pink on exposure to air.
Reactions
Manganese(II) hydroxide is a base exhibiting weak amphoteric behavior. It reacts with acids forming the corresponding manganese(II) salt:
Mn(OH)2 + 2KOH → K2Mn(OH)4
The hydroxide is rapidly oxidized in air forming manganese(III) oxide, Mn2O3.
References
Handbook of Inorganic Chemicals
Chemical Properties
white to pink crystal(s); hardness is 2.5 Mohs [HAW93]