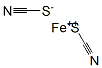Physical Properties
Pale-green monoclinic prisms; unstable; readily oxidized on exposure to air; decomposes on heating; very soluble in water; also soluble in alcohol and ether.
Uses
iron di(thiocyanate) is used as an analytical reagent and as an indicator for detecting peroxides in organic solutions
Preparation
Iron(II) thiocyanate is obtained as a trihydrate by dissolving iron metal in thiocyanic acid in an inert atmosphere in the absence of air.
Chemical Properties
pale green, monoclinic prisms; rapidly oxidized when exposed to air [MER06]
Uses
Iron(II) thiocyanate is used as an analytical reagent and as an indicator for detecting peroxides in organic solutions.