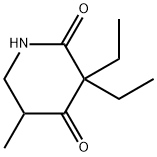
Chemical Properties
Nearly white, crystalline powder; slightcharacteristic odor; bitter taste. Melting range 74–77C. Soluble in water; very soluble in alcohol, chlo-roform, ether, and benzene.
Manufacturing Process
24 parts by weight of powdered sodium are suspended in 100 parts by
volume of absolute benzene and to this suspension is added a freshly
prepared solution of 150 parts by weight of methyl formate and 165 parts by
weight of 2,4-dioxo-3,3-diethyl-piperidine in 900 parts by volume of absolute
benzene. By cooling with cold water, the temperature is maintained at 25° to
28°C. After being stirred for 12 hours 200 parts by volume of 0.6 N sodium
hydroxide are added while cooling. The aqueous layer is separated and
acidified to Congo red by means of 35% hydrochloric acid. The 2,4-dioxo-3,3-
diethyl-5-oxymethylenepiperidine is precipitated in good yield as a solid. After
having been recrystallized in chloroform/petroleum ether it melts at 140° to 141°C.
5 parts by weight of 2,4-dioxo-3,3-diethyl-5-oxymethylene-piperidine are
hydrogenated in 25 parts by volume of methanol in the presence of about 2
parts by weight of Raney nickel at 120°C and under an elevated pressure of
100 atm. Once 2 mols of hydrogen are absorbed, the hydrogenation is
interrupted, the solution is separated from the catalyst and concentrated and
the residue is distilled in vacuo. The distillate, boiling between 178° and
185°C under a pressure of 16 mm, consists of 2,4-dioxo-3,3-diethyl-5-methylpiperidine, which melts at 74° to 75°C.
The same compound is obtained when proceeding according to the following
alternative procedure. A mixture of 39.4 parts by weight of 2,4-dioxo-3,3-
diethyl-5-oxymethylenepiperidine and 27 parts by weight of dibutylamine are
heated to 150°C in a closed vessel. The 2,4-dioxo-3,3-diethyl-5-dibutylaminomethylene-piperidine formed melts at 77°C after having been recrystallized in
petroleum ether.
31 parts by weight of the latter compound are hydrogenated in 150 parts by
volume of alcohol, containing 6 parts by weight of acetic acid, in the presence
of 10 parts by weight of Raney nickel, at 120°C and under an elevated
pressure of 100 atm. The catalyst is separated and the solution is distilled in
vacuo. The 2,4-dioxo-3,3-diethyl-5-methyl-piperidine boils between 178° and
185°C under a pressure of 16 mm and melts at 74° to 75°C.
World Health Organization (WHO)
Methyprylon, a piperidine derivative, was introduced in 1955 for
use as a sedative-hypnotic drug. Habituation, tolerance, physical dependence and
addiction can occur and methyprylon is controlled under Schedule IV of the 1971
Convention on Psychotropic Substances.
(Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV),
, , 1971)