Description
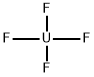
Uranium tetrafluoride, with the molecular formula of UF4, is a green, nonvolatile, crystalline powder that is insoluble in water. It is highly corrosive and is also a radioactive poison.
Preparation
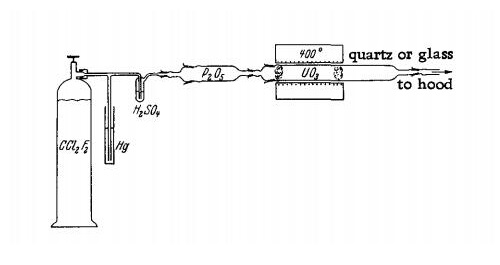
Dichlorodifluoromethane (Freon 12) is passed through a Hg pressure release valve, a bubble counter and aPsO5 tube into a glass or quartz reaction tube (diameter 2.5 cm, length 40 cm.) The reaction tube is inserted into a short electric furnace which can be heated to a temperature of 400℃. Powdered UO3 is placed in the reaction tube between glass-wool plugs. The escaping gases are led to the hood.
At the beginning, dry oxygen is passed through the apparatus for one hour, while the furnace is heated to 400℃. The oxygen flow is then replaced with CF2Cl2, which is introduced at a rate of one liter per hour. The reaction starts as soon as the temperature reaches 400°C. The progress of the reaction can be followed as the color of the product changes to green.
On completion of the reaction, the product is cooled in a stream of CF2Cl2; very pure UF4 is obtained. The yield is almost quantitative.