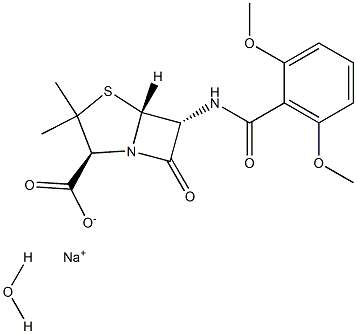
METHICILLIN SODIUM (500 MG) (AS)
- Product NameMETHICILLIN SODIUM (500 MG) (AS)
- CAS7246-14-2
- CBNumberCB5501375
- MFC17H19N2O6S.Na.H2O
- MW420.41261
- MDL NumberMFCD07787409
- MOL File7246-14-2.mol
- MSDS FileSDS
METHICILLIN SODIUM (500 MG) (AS) Chemical Properties,Usage,Production
Description
Methicillin was synthesized independently by Bristol-Myers Laboratories and Beecham Research Laboratories in 1960. It was the first member of the penicillinase-stable semisynthetic penicillin class of antibiotics to be introduced clinically. This antibiotic is a parenteral penicillin having an antibacterial spectrum similar to that of benzylpenicillin. Although its activity against benzylpenicillin-sensitive bacteria is about 1/3 to 1/50 that of benzylpenicillin, it shows strong activity against benzylpenicillinresistant strains because of its stability toward penicillinase. Methicillin has been used for therapy of respiratory tract and urinary tract infections, sepsis, and gynecological and other infections caused by benzylpenicillin-resistant bacteria.Originator
Celbenin ,Beecham,UK,1960Uses
Antibacterial.Definition
ChEBI: Methicillin sodium monohydrate is a hydrate. It contains a methicillin sodium.Manufacturing Process
To a stirred suspension of 6-aminopenicillanic acid (540 g) in dry alcohol-free chloroform (3.75 liters) was added dry triethylamine (697 ml), and the mixture stirred for 10 minutes at room temperature. It was then cooled in a bath of crushed ice while a solution of 2,6-dimethoxybenzoyl chloride (500 g) in dry alcohol-free chloroform (3.75 liters) was added in a steady stream over 20 minutes. When all the acid chloride had been added the cooling bath was removed and the mixture stirred for 1 hour at room temperature. The mixture was stirred vigorously and sufficient dilute hydrochloride acid (2.3 liters of 0.87 N) was added to give an aqueous layer of pH 2.5. The mixture was filtered, the layers separated, and only the chloroform layer was retained.This was stirred vigorously while further dilute hydrochloric acid (0.69 liter of 0.87 N) was added to give an aqueous layer of pH 1. The layers were separated and again only the chloroform layer was retained. Then the chloroform layer was stirred vigorously while sufficient sodium bicarbonate solution (3.2 liters of 0.97 N) was added to give an aqueous layer of pH 6.7 to 7.0. The layers were separated and both were retained. The chloroform layer was stirred vigorously while sufficient sodium bicarbonate solution (50 ml of 0.97 N) was added to give an aqueous layer of pH 7.7, and again the layers were separated. The two bicarbonate extracts were combined, washed with ether (1 liter), and then concentrated at low temperature and pressure until the concentrate weighed 1,415 g.
The concentrate was treated with dry acetone (22 liters), the mixture well mixed, and then filtered to remove precipitated solid impurities. Further dry acetone (4 liters) was added to the filtrate, then the product started to crystallize slowly. Crystallization was allowed to proceed at a temperature between 0° and 3°C for 16 hours and then the product (563 g) was collected by filtration. Dry ether (7.5 liters) was added to the filtrate, and after several hours a second crop (203 g) of solid was collected. The two crops were combined to give sodium 2,6-dimethoxyphenylpenicillin monohydrate (766 g, 73%) as a white crystalline solid.
brand name
Staphcillin (Apothecon).Therapeutic Function
AntimicrobialPreparation Products And Raw materials
Raw materials
METHICILLIN SODIUM (500 MG) (AS) Suppliers
Global(13)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7724 | 58 | |
| 86-21-63210123 | sj_scrc@sinopharm.com | China | 9815 | 79 | |
| 022-6537-8550 15522853686 |
sales@altasci.com.cn | China | 4511 | 55 | |
| +86-400-002-6226 +86-13028896684; |
sales@rrkchem.com | China | 57423 | 58 | |
| 0519-88910898 18661161657 |
yaoxiangqiu2021@163.com | China | 9996 | 58 | |
| 020-81960175-877 18933936954 |
tianwen.zhan@cato-chem.com | China | 7734 | 58 | |
| 13342851930 | 3008394369@qq.com | China | 15726 | 58 | |
| 18153089275 | 3032079623@qq.com | China | 22695 | 58 |
7246-14-2, METHICILLIN SODIUM (500 MG) (AS)Related Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine