Description
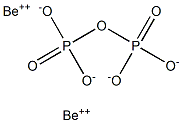
By precipitating a solution of sodium pyrophosphate
with a basic solution of beryllium nitrate, a white pulverable
precipitate was obtained, (BeOH)2H2P2O7. On analysis,
this salt yielded results close to the theoretical
formula for the pyrophosphate. However, the salt was
completely dissolved for analysis and did not take into
account the hydroxy-groups attached to the Be atom.
Once formed, Be2P2O7 has been found to be dimorphic,
the high-temperature form, a-Be2P2O7 being stable
above about 68°C.
Beryllium dihydrogen pyrophosphate is unknown.