Synthesis
Add 30 g (0.112 mol) of methyl 2-chloro-3-methyl-4-methylsulfonylbenzoate and 120 g of chlorobenzene to 500 mLFour reaction bottles; Dissolve 6.8 g (0.045 mol) of sodium bromate in 60 g of water. Obtaining aqueous sodium bromate solution. Dissolving 11.6 g (0.112 mol) of sodium bromide in 60 g of water to obtain an aqueous solution of sodium bromide and adding the obtained aqueous solution of sodium bromate and aqueous sodium bromate to the 500 mL mentioned above four-neck reaction flask; The temperature was raised to 80 ° C with stirring, and 0.93 g (0.006 mol) of azobisisobutyronitrile was added to a 500 mL four-neck reaction flask; At 80 ° C, 19.2 g (0.078 mol) of a 40% aqueous sulfuric acid solution was added dropwise with stirring, and the addition was completed in 3 hours; After the completion of the dropwise addition, the reaction was stirred at 80 ° C for 1 hour.
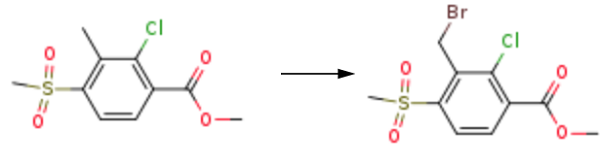
And it was then allowed to stand still while standing to separate the aqueous phase. The organic phase is added with a small amount of sodium hydrogen sulfite aqueous solution to wash once, and after separating the aqueous layer, the organic phase recovers the solvent under negative pressure, and the obtained solid is recrystallized from ethanol.35.9 g of methyl 3-bromomethyl-2-chloro-4-methyl-sulfonylbenzoate was obtained with a purity of 98.1% and a yield of 92.1%.
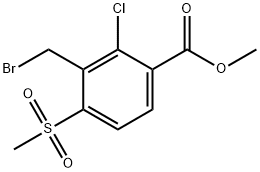
 And it was then allowed to stand still while standing to separate the aqueous phase. The organic phase is added with a small amount of sodium hydrogen sulfite aqueous solution to wash once, and after separating the aqueous layer, the organic phase recovers the solvent under negative pressure, and the obtained solid is recrystallized from ethanol.35.9 g of methyl 3-bromomethyl-2-chloro-4-methyl-sulfonylbenzoate was obtained with a purity of 98.1% and a yield of 92.1%.
And it was then allowed to stand still while standing to separate the aqueous phase. The organic phase is added with a small amount of sodium hydrogen sulfite aqueous solution to wash once, and after separating the aqueous layer, the organic phase recovers the solvent under negative pressure, and the obtained solid is recrystallized from ethanol.35.9 g of methyl 3-bromomethyl-2-chloro-4-methyl-sulfonylbenzoate was obtained with a purity of 98.1% and a yield of 92.1%.