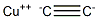Chemical Properties
brown-black solid; explodes [CRC10]
Physical properties
Brownish black powder; insoluble in water.
Uses
Cupric acetylide is used as a detonator.Its applications are very limited, however,because of its high sensitivity to impact orfriction. It is susceptible to form and buildup upon prolong contact of copper metal withorganic vapors.
Preparation
Copper(II) acetylide may be prepared by passing alkyl acetylene vapors over aqueous solution of ammoniacal copper salt.
Hazard
Copper(II) acetylide is highly sensitive to impact, friction or heat. Mild impact or heating can cause a violent explosion. In the dry state it is flammable and is more sensitive to impact or friction than copper(I) acetylide.
Fire Hazard
Cupric acetylide is much more sensitive to
impact and friction than the cuprous salt.
Friction heating or mild impact can result in
violent explosion. In dry state, its sensitivity
is much greater to impact and is flammable.
Safety Profile
Confirmed carcinogen.
Cases of berylliosis have been reported from
exposure to so-called low berylhum alloys.
Human systemic effects by inhalation:
dyspnea, fibrosing alveolitis, weight loss, or
decreased weight gain. See also
BERYLLIUM COMPOUNDS and
COPPER COMPOUNDS. When heated to
decomposition it emits very toxic fumes of
BeO.