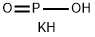Chemical Properties
White, opaque crystals or powder; pun-
gent saline taste; very deliquescent. Soluble in water
and alcohol; decomposed by heat.
Production Methods
Potassium hypophosphite, KH2PO2, white solid, soluble, formed (1) by reaction of hypophosphorous acid and potassium carbonate solution, and then evaporating, (2) by reaction of potassium hydroxide solution and phosphorus on heating (poisonous phosphine gas evolved).
Hazard
Moderate fire risk, may explode if ground
with chlorates, nitrates, or other strong oxidizing
agents.
Flammability and Explosibility
Not classified