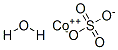Chemical Properties
Reddish crystalline powder
Uses
Cobalt(II) sulfate hydrate (CoSO4.H
2O) can be used in the synthesis of coordination polymers. It can also be used as a starting material in the synthesis of cobalt sulfides hexagonal nanocrystallites.
Uses
Ceramics, pigments, glazes, plating baths, catalyst, storage batteries, paint/ink drier
Purification Methods
Crystallise it three times from conductivity water (1.3mL/g) between 100o and 0o depending on which hydrate is required. The heptahydrate crystallises below 44o and is efflorescent with m 97o . Between 44o and 70o the monoclinic hexahydrate CoSO4.6H2O m 41.5o is formed, and above 70o the monohydrate CoSO4.H2O m 71o is obtained. The pale reddish or lavender-coloured anhydrous salt is obtained by heating the hydrate above 250o, boiling with conc H2SO4 or heating with (NH4)2SO4).