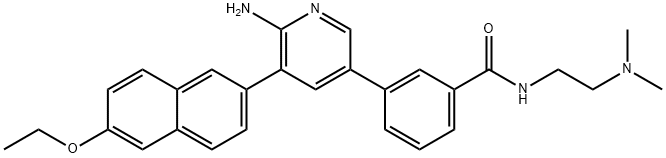
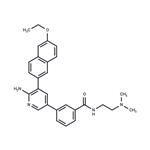
Description
Protein kinase D (PKD) is a serine/threonine protein kinase that is activated by diacylglycerol, commonly downstream of PKC signaling. The three human PKD isoforms target a variety of proteins to alter cell proliferation, survival, invasion, and protein transport. CRT5 is a pyrazine benzamide that prevents activation of all three isoforms of PKD in endothelial cells treated with VEGF (IC
50s = 1, 2, and 1.5 nM for PKD1, PKD2, and PKD3, respectively). It has little effect on a panel of additional kinases when given at 1 μM. CRT5 blocks phosphorylation of PKD1 on Ser
916 and PKD2 on Ser
876, but does not affect PKC-dependent PKD phosphorylation or PKD autophosphorylation. It also decreases VEGF-induced endothelial migration, proliferation, and tubulogenesis.
in vitro
the non-linear regression analysis revealed that ld50 value of crt5 was 17 μm. the biochemical ic50 value of crt5 for pkd1, pkd2 and pkd3 were 1, 2 and 1.5 nm, respectively [1]. crt5 (1 μm) completely inhibited pkd1 and pkd2, but showed little inhibitory effect on the pkc isoforms. crt5 significantly reduced vegf-induced phosphorylation of hsp27 at the position ser82. crt5 significantly reduced the migratory response towards vegf by 42–51%. crt5 decreased the proliferation of control cells not treated with vegf to a less extent. vegf increased huvec tubule formation in a collagen-based assay. crt5 markedly inhibited vegf-induced tubulogenesis [1].
References
[1] evans i m, bagherzadeh a, charles m, et al. characterization of the biological effects of a novel protein kinase d inhibitor in endothelial cells[j]. biochemical journal, 2010, 429(3): 565-572.