Health Benifits
7,9-Ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione belongs to flavonoid compound. It can be found in morels (Morchella spp.), which are among the most valuable and important mushrooms because of their taste and commercial value.
It can be identified as one of bioactive compounds in whole plant extract of Euphorbia pulcherrima, which are used in folk medicine to treat skin diseases, gonorrhea, migraine, intestinal parasites, and warts.
In addition, 7,9-ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione can also be found in marine algae, which have been highly acknowledged to possess noticeable pharmacological activities, including antineoplastic, antimicrobial and antiviral activities due to their specific functional compounds (which are not available in other plants).
Chemical Properties
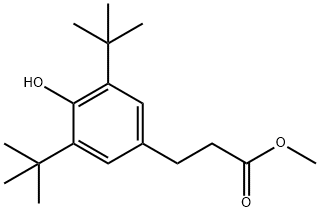
7,9-Ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione (C17H24O3, CAS registry No. 82304-66-3) is an oxaspiro compound that is 1-oxaspiro[4.5]deca-6,9-diene-2,8-dione carrying two additional tert-butyl substituents at positions 7 and 9. It is an oxaspiro compound with a lactone, an enone and a cyclic ketone. Its melting point is 139-140 °C.
Uses
7,9-Di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione is an antioxidant naturally found in aerial parts of Gmelina asiatica Linn (Verbenaceae). It is also found in essential oils of some Stachys species from Mediterranean area. It is an impurity of Irganox 1098 (A697523) used in the food packaging.
Definition
ChEBI: 7,9-di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione is an oxaspiro compound that is 1-oxaspiro[4.5]deca-6,9-diene-2,8-dione carrying two additional tert-butyl substituents at positions 7 and 9. It is an oxaspiro compound, a lactone, an enone and a cyclic ketone.
![7,9-ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione Structure](https://www.chemicalbook.com/CAS/20180703/GIF/82304-66-3.gif)