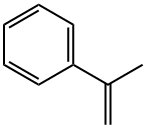
Description
Methylstyrene is a colorless liquid with a characteristic odor. Molecular weight = 118.19; Specific gravity(H2O:1) 5 0.91; Boiling point = 165.6℃; Freezing/Meltingpoint 5 2 23.3℃; Vapor pressure 5 2 mmHg at 20℃;Flash point = 54℃; Autoignition temperature 5 574℃.Explosive limits: LEL 5 1.9%; UEL 5 6.1%. HazardIdentification (based on NFPA-704 M Rating System):Health 1, Flammability 2, Reactivity 0. Insoluble in water.Potential Exposure: Tumorigen,Mutagen, Human Data; Primary Irritant. Methylstyrene isused as an additive, a plasticizer, and as a copolymer; usedin the production of modified polyester and alkyd resinformulations.
Chemical Properties
Methylstyrene is a colorless liquid with a characteristic odor.
Chemical Properties
clear colourless liquid
Physical properties
Colorless liquid with a sharp aromatic odor. Odor threshold concentration is 290 ppb (quoted,
Amoore and Hautala, 1983).
Uses
α-Methylstyrene is not a styrenic monomer in the strict sense. The methyl substitution on the side chain, rather than the aromatic ring, moderates its reactivity in polymerization. It is used as a specialty monomer in ABS resins, coatings, polyester resins, and hot-melt adhesives. As a copolymer in ABS and polystyrene, it increases the heat-distortion resistance of the product. In coatings and resins, it moderates reaction rates and improves clarity.
Uses
Intermediate for ABS plastics, Styrene - Butadiene rubber, Polystyrene, Styrene - Acrylonitrile Resins, Perfumery, Polyalphamethyl Styrene, Polyester resins
Uses
Polymerization monomer, especially forpolyesters.
Production Methods
α-Methylstyrene (AMS) is produced as a by-product in the production of phenol and acetone from cumene.
Definition
ChEBI: Alpha-Methylstyrene is an olefinic compound.
General Description
A colorless liquid. Insoluble in water and less dense than water. Flash point 115°F. May be mildly toxic by ingestion, inhalation and skin absorption. Vapors may be narcotic by inhalation. Used as a solvent and to make other chemicals.
Air & Water Reactions
Flammable. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. Insoluble in water.
Reactivity Profile
2-Phenyl-1-propene is easily peroxidizable; the peroxide may initiate exothermic polymerization of the bulk material. Reacts violently with strong oxidizing agents [Handling Chemicals Safely, 1980 p. 866; Bretherick, 1979 p. 160].
Hazard
Moderate fire risk. Explosive limits in air1.9–6.1%. Avoid inhalation and skin contact. Upperrespiratory tract irritant, kidney and female repro-ductive damage. Possible carcinogen.
Health Hazard
Inhalation causes irritation of respiratory tract, headache, dizziness, light-headedness, and breathlessness. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes. Prolonged skin contact can cause severe rashes, swelling, and blistering.
Safety Profile
Mildly toxic by
inhalation. Human systemic effects by
inhalation: irritant effects. A skin and eye
irritant. Flammable when exposed to heat or
flame; can react vigorously with oxidizing
materials. When heated to decomposition it
emits acrid smoke and irritating fumes.
Potential Exposure
Methylstyrene is used as additive,
plasticizer, and copolymer; used in the production of
modified polyester and alkyd resin formulations.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Chemical/Physical. Polymerizes in the presence of heat or catalysts (Hawley, 1981).
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Store intightly closed containers in a cool, well-ventilated areaaway from oxidizers. Where possible, automatically pumpliquid from drums or other storage containers to processcontainers.
Shipping
UN2303 Isopropenylbenzene, Hazard Class: 3;
Labels: 3-Flammable liquid
Purification Methods
Wash the monomer three times with aqueous 10% NaOH (to remove inhibitors such as quinol), then six times with distilled water, dry with CaCl2 and distil it unde
Incompatibilities
Vapors may form explosive mixture
with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong bases, strong acids, oxoacids,
epoxides, peroxides, halogens, catalysts for vinyl or ionic
polymers; aluminum, iron chloride; copper. Methylstyrene
may form unstable peroxides; may polymerize. Usually
contains an inhibitor, such as tert-butyl catechol.
Toxics Screening Level
The current ITSL for Alpha-Methyl Styrene is 230 μg/m3, with annual averaging time (AT).
Waste Disposal
ncineration, often by admixture with a more flammable solvent