Description
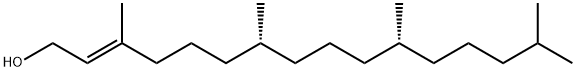
Phytol is a diterpene alcohol obtained from the degradation of chlorophyll and has been used in the synthesis of Vitamins E and K. During the digestion process of ruminants, phytol is liberated from chlorophyll and converted to phytanic acid to be stored in fats. While humans cannot derive phytol from chlorophyll, free phytol, obtained from the consumption of ruminant adipose tissue and dairy products, is readily absorbed in the small intestine and converted to phytanic acid. Phytanic acid accumulates to toxic levels in a number of metabolic disorders, and the conversion of phytol to phytanic acid has been shown to be regulated
via the activation of peroxisome proliferator-activated receptor α (PPARα). Phytol and its metabolites have also been reported to activate retinoid X receptors (RXRs; K
is range from 2.3-67.2 μM) and to promote the activity of PPAR/RXR heterodimers. Phytol also demonstrates sedative and anxiolytic effects through interaction with the GABA
A receptor, and it has been explored as an antischistosomal agent in a mouse model of schistosomiasis.
Chemical Properties
Colorless to yellow viscous liquid with a faint floral scent, found naturally in jasmine essential oil and tea. It cannot dissolve in water, but is able to dissolve in organic solvents.
Occurrence
Reported found in Herniaria incana lam oil Greece (2.60%) and Witch hazel leaf oil (9.79%).
Uses
trans-Phytol is a reagent that is used in the preparation of α-tocopherol analogs as mitochondrial antioxidants. It is also used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1.
Definition
ChEBI: Phytol is a diterpenoid that is hexadec-2-en-1-ol substituted by methyl groups at positions 3, 7, 11 and 15. It has a role as a plant metabolite, a schistosomicide drug and an algal metabolite. It is a diterpenoid and a long-chain primary fatty alcohol.
Production Methods
Phytol can be obtained from natural raw materials by a variety of processes. The industrial synthesis of phytol starts from isophytol. The latter is first treated with formic acid to give phytyl formate, from which (Z, E )-phytol (isomer ratio 3 : 7) is obtained by saponification or transesterification.
Aroma threshold values
Low strength odor, floral type.
Safety Profile
Low toxicity by
ingestion and skin contact. A skin irritant.
When heated to decomposition it emits
acrid smoke and irritating fumes.