Description
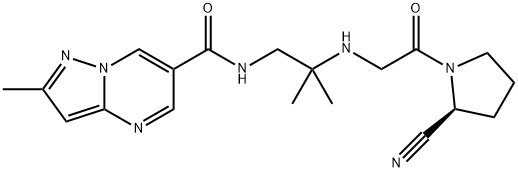
Anagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that was approved
in Japan in November 2012 for the treatment of patients with Type 2 diabetes
mellitus (T2DM).
Anagliptin (also known asSK-0403) is a treatment for diabetes based on inhibition of DPP-4, an enzyme that is responsible for degradation of glucagon-like peptide 1 (GLP-1), a 30-amino acid peptide that is secreted in response to food intake. GLP-1 stimulates
insulin secretion and inhibits glucagon secretion, which leads to lower
levels of plasma glucose. Following the introduction of the first DPP-4 inhibitor, sitagliptin, in 2006, several members of the gliptin class have been approved worldwide. Anagliptin was discovered from an effort to replace
a metabolically labile isoindoline group from an earlier DPP-4 inhibitor series with a stable bioisostere. Anagliptin is a potent DPP-4 inhibitor, with an IC
50=3.8 nM and >10,000-fold selectivity over inhibition of DPP-8 and DPP-9.
Originator
Sanwa Kagaku Kenkyusho (Japan)
Uses
Anagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction. It also attenuates atherosclerosis in male apolipoprotein E-deficient mice.
Definition
ChEBI: Anagliptin is an amino acid amide.
Clinical Use
Anagliptin, which is marketed as Beskoa or Suiny, is a dipeptidyl peptidase–IV (DPP-4) inhibitor
which was approved in September 2012 and launched in November 2012 in Japan for the treatment of
Type II diabetes. The drug was co-developed by three Japanese companies; Kowa, Sanwa Kagaku and
JW pharmaceutical. Anagliptin, which is more selective against several recombinant human proteases by comparison to sitagliptin and vildagliptin, has more than 10,000-fold selectivity over the
structurally homologous DPP-8 and DPP-9 enzymes.
Synthesis
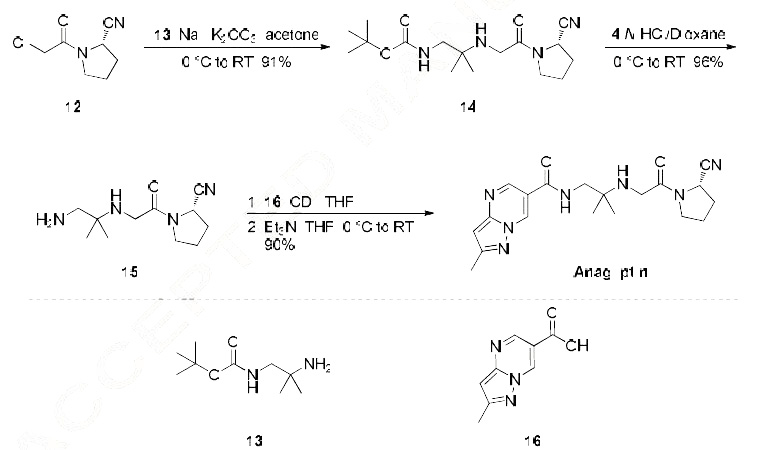
The most likely process-scale synthesis has been published and is depicted in Scheme 3.24
Commercially available (S)-1-(2-chloroacetyl)-pyrrolidine-2-carbonitrile (12) was alkylated with t-butyl
(2-amino-2-methyl-1-propyl)carbamate (13), giving rise to (S)-t-butyl (2-((2-(2-cyanopyrrolidin-1-yl)-2-
oxoethyl)amino)-2-methylpropyl)carbamate (14). This Boc-protected system was subsequently treated
with strong acid to give the ethylene diamine derivative 15 in 96% yield. Activation of 15 with CDI
followed by coupling with commercially available 2-methylpyrazolo[1,5-a] pyrimidine-6-carboxylic
acid (16) gave anagliptin (III) in 90% yield.