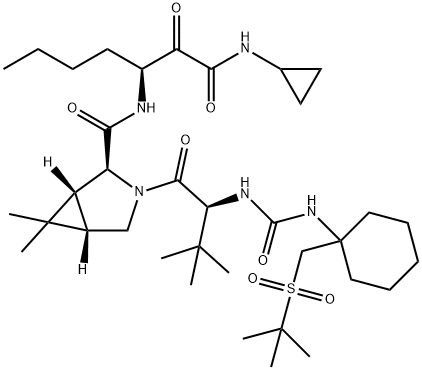
Narlaprevir
- Product NameNarlaprevir
- CAS865466-24-6
- CBNumberCB52554290
- MFC36H61N5O7S
- MW707.96
- MDL NumberMFCD16038932
- MOL File865466-24-6.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 152 - 155°C |
| Density | 1.21 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.84±0.20(Predicted) |
| color | White to Off-White |
| Stability | Hygroscopic |
| FDA UNII | 2857LA2O07 |
Narlaprevir Chemical Properties,Usage,Production
Description
Narlaprevir was approved as a treatment for genotype 1 HCV and serves as a class 2 HCV NS3 serine protease inhibitor. In clinical trials, it showed a rapid and steady decline in HCV-RNA levels in both previously treated and treatment-naive patients when used in combination with ritonavir and PEG-IFN-α. This combination ultimately led to ≥50% of patients with undetectable HCV-RNA levels after a second period of treatment. Narlaprevir also has demonstrated activity against HCV mutations resistant to other treatments such as boceprevir and telaprevir. The unique activity of this drug can be attributed to a critical electrophilic α-keto-amide “warhead”, which covalently reacts with an HCV NS3 protease active-site serine residue involved in the HCV viral replication process. Because of their essential roles in viral replication, HCV NS3 and NS5B proteases have recently become key targets for HCV drug development. Strategically, the development of narlaprevir stems specifically from the pursuit of a single-diastereomer, second generation HCV protease inhibitor, which would provide in vitro potency and pharmacokinetic profile improvements over the structurally related antiviral drug boceprevir,which exists as a mixture of diastereomers. After the R-Pharm pharmaceutical group obtained the license to manufacture narlaprevir from Merck in 2012, further development of the drug was realized through collaborations with Schering-Plough and Texas Liver Institute.Uses
Narlaprevir is an NS3/4A protease inhibitor used in the treatment of hepatitis C virus, HCV.Definition
ChEBI: Narlaprevir is an azabicyclohexane that is (1R,5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane substituted by [(3S)-1-(cyclopropylamino)-1,2-dioxoheptan-3-yl]aminoacyl and N-({1-[(tert-butylsulfonyl)methyl]cyclohexyl}carbamoyl)-3-methyl-L-valyl groups at positions 2S and 3, respectively. It is a hepatitis C virus (HCV) NS3/4A serine protease inhibitor (Ki = 6 nM) that is used for the treatment of chronic hepatitis C. It has a role as a hepatitis C protease inhibitor, an antiviral drug, an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor and an anticoronaviral agent. It is a sulfone, a member of ureas, a tertiary carboxamide, an azabicyclohexane, a pyrrolidinecarboxamide, a secondary carboxamide and a member of cyclopropanes.Synthesis
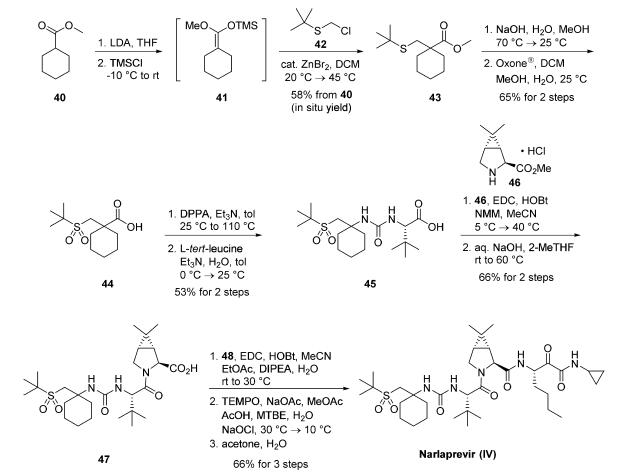
A kilogram-scale synthetic route to narlaprevir has been reported and proceeds strategically through the union of urea 45, bicyclic amine intermediate 46, and amine salt 48 . Preparation of urea 45 begins with commercial cyclohexanecarboxylic acid methyl ester (40), which was treated with freshly prepared LDA and TMSCl in THF to provide silyl enol ether 41. This intermediate was immediately reacted with commercial 2-[(chloromethyl)thio]- 2-methylpropane (42) under Lewis acid conditions (ZnBr2) to provide ester 43 in 58% yield over the two-step process.17,19 A solution of crude 43 was subjected to saponification conditions (NaOH, H2O, MeOH) and sulfide oxidation with oxone in DCM/MeOH, leading to the target sulfone 44 in 65% yield. From 44, a Curtius rearrangement delivered an isocyanate intermediate that could be trapped with L-tert-leucine, forming the desired urea 45 in 53% over the two-step sequence.17,19 Coupling 45 with commercially available bicyclic amine 46 under peptide coupling conditions (EDC, HOBt, NMM) led to the desired amide in 79% yield, which was then saponified with aqueous NaOH in 2-methyltetrahydrofuran (2-MeTHF) to provide acid intermediate 47 (84% yield). This intermediate was coupled with amine salt 48 with EDC and HOBt, providing the penultimate intermediate to narlaprevir. Completion of the synthesis relied upon installation of the essential |á-keto-amide functionality, which was accomplished by |á-hydroxy amide oxidation using TEMPO-catalyzed conditions. A final recrystallization from acetone/water completed synthesis of narlaprevir (IV) in 83% yield. It is worth noting that this overall route was used to generate >1 kg of narlaprevir and required no chromatographic separation steps.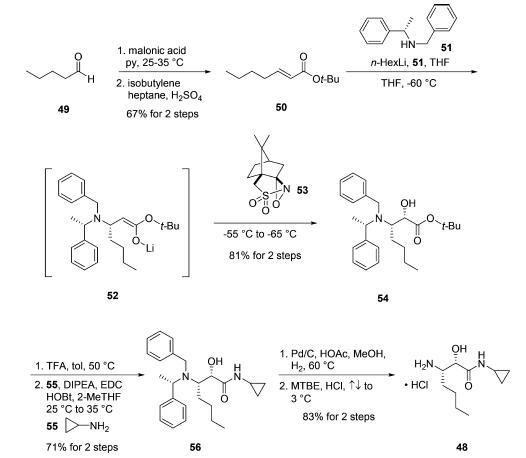
Amine salt 48 was prepared by first subjecting commercially available pentanal (49) to Knoevenagel condensation conditions using malonic acid followed by conversion of the resulting acid to the corresponding t-butyl ester 50 by reaction with H2SO4 and isobutylene. The key transformation for establishing the requisite stereocenter in intermediate 48 relied on an asymmetric conjugate addition of a bis-protected lithiated amine followed by enolate trap with an electrophilic source of oxygen. In practice, treatment of |á- methyl-N-(phenylmethyl)-(|áS)-benzenemethanamine (51) with n-hexyllithium resulted in stereoselective 1,4-addition to enone 50. Subjection of lithium enolate intermediate 52 to (1S)-(+)-(10-camphorsulfonyl)oxaziridine (53) then furnished the |á-hydroxyl group and delivered the syn-amino alcohol derivative 54 in 81% yield for the two-step protocol. tert-Butyl ester removal was realized by exposure of 54 to TFA in warm toluene. Subsequent coupling of the resulting acid with cyclopropylamine (55) utilizing EDC and HOBt conditions provided cyclopropyl amide 56 in 71% yield from 54. Finally, hydrogenolytic removal of the benzyl groups from the |?-amine followed by subjection of the product to refluxing HCl provided amine salt 48 in 83% yield.19a
target
NS3 proteaseReferences
[1]. arasappan a, bennett f, bogen s l, et al. discovery of narlaprevir (sch 900518): a potent, second generation hcv ns3 serine protease inhibitor. acs medicinal chemistry letters, 2010, 1(2): 64-69.[2]. tong x, arasappan a, bennett f, et al. preclinical characterization of the antiviral activity of sch 900518 (narlaprevir), a novel mechanism-based inhibitor of hepatitis c virus ns3 protease. antimicrobial agents and chemotherapy, 2010, 54(6): 2365-2370.
[3]. wang h, geng l, chen b z, et al. computational study on the molecular mechanisms of drug resistance of narlaprevir due to v36m, r155k, v36m+ r155k, t54a, and a156t mutations of hcv ns3/4a protease. biochemistry and cell biology, 2014, 92(5): 357-369.
Preparation Products And Raw materials
Narlaprevir Suppliers
Global(50)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +8618523575427 | sales@conier.com | China | 49732 | 58 | |
| 021-31761238 | sales@saferph.com | CHINA | 897 | 58 | |
| +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32159 | 58 | |
| +86-0371-86658258 +8613203830695 |
laboratory@coreychem.com | China | 30231 | 58 | |
| 0917-3909592 13892490616 |
gksales1@gk-bio.com | China | 9306 | 58 | |
| +86-0551-65418684 +8618949823763 |
sales@tnjchem.com | China | 25356 | 58 | |
| +86-25-84696168 +86-15380713688 |
Austin@fredbio.com | China | 2428 | 58 | |
| +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 | |
| +86-18621343501; +undefined18621343501 |
product@acmec-e.com | China | 33338 | 58 | |
| +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
865466-24-6, NarlaprevirRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine

