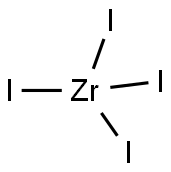Description
Zirconium(IV) iodide, ZrI4, is the most readily available iodide of zirconium. It is an orange-colored solid that degrades in the presence of water. The compound was once renowned as an intermediate in the purification of zirconium metal. This compound is volatile, subliming as intact tetrahedral ZrI4 molecules.
Chemical Properties
orange crystal(s); fumes heavily in air; cub, a=1.179nm [KIR84] [MER06]
Uses
Used in the van Arkel-de Boer refining process.
Preparation
It is prepared by the direct reaction of powdered zirconium metal and iodine. Pyrolysis of zirconium(IV) iodide gas by contact of hot wire was the first industrial process for the commercial production of pure ductile metallic zirconium.
reaction suitability
reagent type: catalyst
core: zirconium
Structure and conformation
Like most binary metal halides, it has a polymeric structure. The compound exists as a polymer consisting of octahedral Zr(IV) centers, each with a pair of terminal iodide ligands and four doubly bridging iodide ligands. The Zr-I distances are 2.692 (terminal) and 3.030 A? .