Uses
Hydrochloride salt of 3-[(1,2-Dichlorovinyl)thio]-L-alanine, a mutagenic and nephrotoxic metabolite of trichloroethylene.
Uses
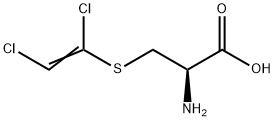
S-(1,2-Dichlorovinyl)-L-cysteine (DCVC) is a model nephrotoxicant
and cataractogen used to induce acute renal failure
and cataracts in experimental animals to study the biochemical,
physiological, and molecular mechanisms underlying the
disease.
Definition
ChEBI: An L-alpha-amino acid that is L-cysteine in which the hydrogen attached to the sulfur is replaced by a 1,2-dichlorovinyl group.
Environmental Fate
Human exposure to DCVC occurs only via potential formation
of DCVC in vivo following trichloroethylene (TCE) exposure;
therefore, no data are available in this regard.
Toxicity evaluation
Cell death is initiated by the metabolism of DCVC via renal
cysteine conjugate b-lyase to a sulfur-containing reactive thiol
radical that covalently binds to macromolecules.
The findings that the nephrotoxicity and cataractogenesis of
DCVC can be blocked by aminooxyacetic acid (a selective
inhibitor of b-lyase) and probenecid (organic anion transport
Encyclopedia of inhibitor) provide evidence for the roles of cysteine conjugate
b-lyase and the organic anion transport system, respectively,
in DCVC-induced nephrotoxicity. Although the b-lyase
enzyme is considered to be the major bioactivating enzyme
for DCVC, other bioactivating enzyme activities
have been described, and some of these may have relevance to
risk assessment. Studies have shown that renal FMO3 can also
metabolize DCVC to form DCVC sulfoxide thereby causing
nephrotoxicity. Reports from several laboratories indicate that
the cytotoxicity of DCVC is mediated at the mitochondrial
level. Depletion of GSH, mitochondrial lipid peroxidation
and GSSG formation, inhibition of mitochondrial
lipoyl dehydrogenase activity, release of Ca
2+ from mitochondria,
and inhibition of mitochondrial membrane
potential have been observed prior to renal cell death and
correlated well with cytotoxicity.