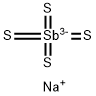Synthesis
A solution of 15 g. of SbCl3
in 600 ml. of dilute hydrochloric
acid is prepared. If a precipitate is produced as a consequence
of hydrolysis, concentrated hydrochloric acid is added until the
solution becomes clear. Then H2S is bubbled through the solution,
and the precipitate of Sb2S3 is filtered off. It is mixed with 60 ml.
of 20% NaOH; 6 g. of S (powder form) is added and the mixture is
boiled with constant stirring until the orange-red color turns
yellow. The water lost on boiling should be replaced from time
to time. The solution is filtered through a fluted filter and evaporated until crystallization begins. If the solution becomes
turbid, a few drops of 20% NaOH are added until it clears. After
complete cooling, the crystalline precipitate is suction-filtered,
washed with some alcohol, and dried in a desiccator over quicklime
to which a few drops of ammonium sulf ide solution have been added.
The mother liquor can be further concentrated. The preparation
can be purified by recrystallization from weakly alkaline solution
(a few milliliters of sodium hydroxide are added to the water).
Sb2S3 + 8 NaOH + 6 S = 2 Na3SbS4 + Na2SO4 + 4 H2O