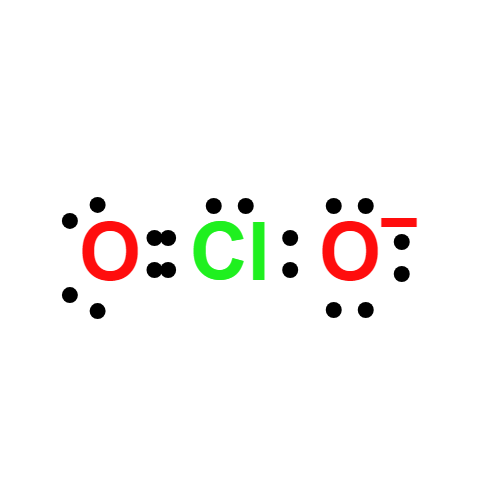
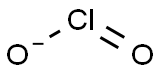Definition
An acid known only in solution and as its salts.
General Description
Crystalline solids or aqueous solutions of these solids. Solids are likely to be water soluble and are denser than water.
Air & Water Reactions
Probably water soluble.
Reactivity Profile
Chlorite are oxidizing agents. May liberate oxygen if heated. May react on contact with organic materials such as oil, grease, wood, etc. The reaction may generate sufficient heat to start a fire. Can react with ammonia to give ammonium chlorite, which is shock-sensitive. Mixtures with finely divided metallic or organic substances are highly flammable and may be ignited by friction, Lab. Gov. Chemist(1965).
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution.
Fire Hazard
May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard.