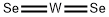Chemical Properties
-325 mesh black powder(s); dry, solid lubricant with exceptional stability at high temperatures, and in high vacuum; used in the form of 99.8% pure material as a sputtering target to produce lubricant films [HAW93] [CER91] [AES93]
Uses
Tungsten(IV) selenide is used as an important compound in electrochemical solar cells. It acts as a stable semiconductor.
Uses
Owing to the thickness dependent physical and chemical properties, the two dimensional layered inorganic nanomaterials have drawn significant attention recently. They find applications in photocatalysis, phtotovoltaics, gas sensing, lithium ion batteries, field effect transistors, spintronics etc. Transition metal dichalcogenides represent an important class of inorganic 2D materials having aforementioned applications. The CVD grown monolayer films of MoS
2, MoSe
2, WS
2 and WSe
2 on insulating substrates such as SiO
2 and sapphire are well suited for electronic and photovoltaic devices.
Uses
The two dimensional layered inorganic nanomaterials have drawn significant attention recently owing to their thickness dependent physical and chemical properties. They find applications in photocatalysis, phtotovoltaics, gas sensing, lithium ion batteries, field effect transistors, spintronics etc. Transition metal dischalcogenides represent an important class of inorganic 2D materials having the aforementioned applications.
Definition
Lamellarstructured,
dry, solid lubricant with exceptionally
high temperature and high vacuum stability.
Retains its lubricity to temperatures as low as
?265C.
Structure and conformation
Tungsten diselenide, WSe2, has a hexagonal crystalline structure comparable to MoS2. Every tungsten atom is covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere, and each selenium is bonded to three
tungsten atoms in a pyramidal geometry. Heating thin films of tungsten under pressure from gaseous selenium and high
temperatures ( . 527℃/800K) using the sputter deposition technique leads to the films crystallizing in hexagonal structures with the correct stoichiometric ratio.
W+ 2Se→WSe2