Preparation
Treatment of poly(vinyl alcohol) with aldehydes or ketones results in the
formation of poly(vinyl acetal)s and poly(vinyl ketal)s, of which only the
former products are of commercial significance. At the present time,
poly(vinyl formal) and poly(vinyl butyral) are the most important members of
this group; poly(vinyl acetal) itself now finds little application.
The preparation of a poly(vinyl acetal) from poly(vinyl alcohol) may be
represented as follows:
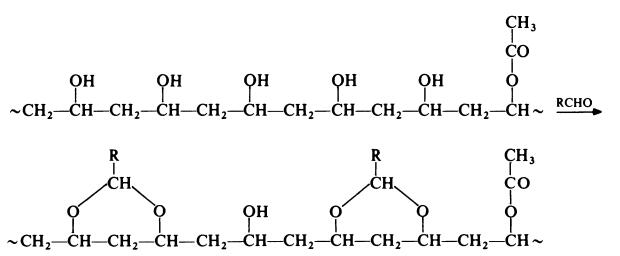
Thus, in practice, a poly(vinyl acetal) contains acetal groups, hydroxyl groups
(which become isolated and cannot undergo reaction with the aldehyde) and
acetate groups (which are due to incomplete hydrolysis of the parent
poly(vinyl acetate)). Differing proportions of these groups lead to various
grades of materials.
Industrial uses
These are relatively soft, water-insoluble thermoplasticproducts obtained by the reaction ofpolyvinyl alcohol with aldehydes. Propertiesdepend on the extent to which alcohol groupsare reacted. Polyvinyl butyral is rubber andtough and is used primarily in plasticized formas the inner layer and binder for safety glass.Polyvinyl formal is the hardest of the group. Itis used mainly in adhesive, primer, and wirecoatingformulations, especially when blendedwith a phenolic resin.
Polyvinyl butyral is usually obtained by thereaction of butyraldehyde with polyvinyl alcohol.The formal can be produced by the sameprocess, but is more conveniently obtained bythe reaction of formaldehyde with polyvinylacetate in acetic acid solution.