Originator
Monodral,Winthrop,US,1954
Manufacturing Process
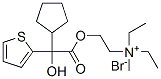
An aqueous solution of 13.8 g of 2-diethylaminoethyl chloride hydrochloride was neutralized with sodium hydroxide, and the free 2-diethylaminoethyl chloride was extracted with ether. The ether extracts were dried over anhydrous magnesium sulfate, filtered, and the filtrate was added to a solution of 13.6 g of cyclopentyl-(α-thienyl)hydroxyacetic acid in 100 ml of isopropyl alcohol. The mixture was then distilled through a 25-cm Vigreauxtype column until the temperature of the vapors reached 80°C. The residual solution was refluxed overnight and then transferred to a beaker along with 350 ml of isopropyl alcohol. The crystalline hydrochloride had meanwhile separated out, and this was filtered, washed with isopropyl alcohol, ether and then dried, giving 23 g, melting point 172°C to 173.5°C. Recrystallization from 400 ml of isopropyl alcohol gave 20.3 g of 2-diethylaminoethyl cyclopentyl-(α-thienyl)hydroxyacetate hydrochloride, melting at 174°C to 175°C; deep yellow-orange color with concentrated sulfuric acid.
The hydrochloride may then be converted to the methobromide by reaction with methyl bromide.
Safety Profile
Poison by intravenous and subcutaneous routes. Moderately toxic by ingestion. When heated to decomposition it emits very toxic fumes of NH3, NOx, SOx, and Br-