Synthesis
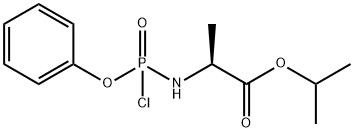
GENERAL STEPS: In a reaction flask equipped with a mechanical stirrer, a reflux condenser tube, a thermometer, and under nitrogen protection, a solution of (L)-alanine isopropyl ester hydrochloride (Q) (2.8 g, 16.8 mmol) prepared in Example 1 was added with methylene chloride (21 mL). The mixture was stirred at room temperature until a homogeneous suspension was formed. Subsequently, the mixture was cooled to -65°C to -55°C using a dry ice-acetone bath and triethylamine (3.9 g, 38.8 mmol) was added slowly, ensuring that the temperature was maintained below -50°C. The mixture was then cooled to -65°C to -55°C using a dry ice-acetone bath. After addition, cooling was continued to -65°C to -55°C and a solution of dichloromethane (11 mL) of phenyl dichlorophosphate (3.3 g, 15.6 mmol) was added dropwise, controlling the temperature to below -50°C. The reaction mixture was stirred at -65°C to -55°C until the reaction was complete (about 1 h).
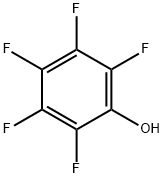
In another reaction flask equipped with the same apparatus, pentafluorophenol (2.4 g, 13.0 mmol) and dichloromethane (28 mL) were added. The mixture was cooled to 0°C to 5°C and triethylamine (1.6 g, 15.8 mmol) was added slowly, keeping the temperature below 10°C. After the addition, the temperature was raised to 20°C to 25°C and maintained for 1 h. It was then added to the previously prepared mixture, which had to be cooled down to -15°C to -10°C and ensuring that the internal temperature did not exceed 5°C. After the addition, the reaction mixture was After addition, the reaction mixture is warmed to 0°C to 5°C and maintained until the reaction is complete (about 1 hour).
Water (8.7 mL) was added to the reaction system and the temperature was controlled to be below 10°C. The reaction was then warmed to 20°C to 25°C, partitioned, and the organic phase was washed with water and concentrated to dryness under reduced pressure. The residual solvent was removed by azeotropic isopropyl acetate to give 9.0 g of residue (quantitative yield) with diastereoisomer ratio Sp:flp = 50:50.
References
[1] Patent: WO2016/151542, 2016, A1. Location in patent: Page/Page column 23; 24
[2] Patent: WO2014/62596, 2014, A1. Location in patent: Page/Page column 60
[3] Patent: US2014/206640, 2014, A1. Location in patent: Paragraph 0286; 0287
[4] Patent: CN106543253, 2017, A. Location in patent: Paragraph 0086-0089
[5] Patent: WO2018/48937, 2018, A1. Location in patent: Page/Page column 220; 221
![L-Alanine, N-[(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphinyl]-, 1-Methylethyl ester Structure](https://www.chemicalbook.com/CAS/20150408/GIF/1256490-52-4.gif)
![L-Alanine, N-[(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphinyl]-, 1-Methylethyl ester](/CAS/20150408/GIF/1256490-52-4.gif)
