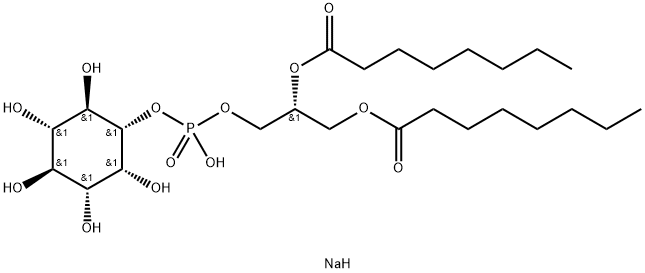
Description
The phosphatidylinositol (PtdIns) phosphates represent a small percentage of total membrane phospholipids. However, they play a critical role in the generation and transmission of cellular signals.
1,2 PtdIns-
(1,2-
dioctanoyl) is a synthetic analog of natural phosphatidylinositol (PtdIns) containing C8:0 fatty acids at the
sn-
1 and
sn-
2 positions. The compound features the same inositol and diacyl glycerol (DAG) stereochemistry as that of the natural compound. The short fatty acid chains of this analog, compared to naturally-
occurring PtdIns, gives it different physical properties including high solubility in aqueous media. PtdIns are phosphorylated to mono-
(PtdIns-
P; PIP), di-
(PtdIns-
P
2; PIP
2), and triphosphates (PtdIns-
P
3; PIP
3). Hydrolysis of PtdIns-
(4,5)-
P
2 by phosphoinositide (PI)-
specific phospholipase C generates inositol triphosphate (IP
3) and DAG which are key second messengers in an intricate biochemical signal transduction cascade.
References
1. Exton, J.H.
Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins Annu. Rev. Pharmacol. Toxicol. 36,481-509(1996).
2. Majerus, P.W.
Inositol phosphate biochemistry Annu. Rev. Biochem. 61,225-250(1992).