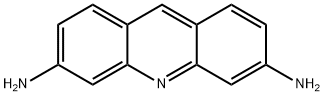
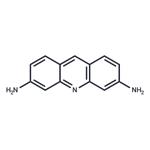
Definition
ChEBI: An aminoacridine that is acridine that is substituted by amino groups at positions 3 and 6. A slow-acting bacteriostat that is effective against many Gram-positive bacteria (but ineffective against spores), its salts were formerly used for treatment of bur
s and infected wounds.
Safety Profile
Poison by intravenous,
intraperitoneal, and subcutaneous routes.
Questionable carcinogen. Mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx. See also other
diaminoacridine entries.
Enzyme inhibitor
This dye (FWfree-base = 209.25 g/mol; CAS 92-62-6; orange-red solid; M.P. = 281 or 288°C; soluble in water; pKa = 9.65; solutions are light sensitive; insoluble in organic solvents such as benzene, diethyl ether, and chloroform), also known as proflavin and 3,6-acridinediamine, is a mutagen that intercalates in double-stranded DNA, inhibiting both DNA and RNA biosynthesis. Proflavine can also participate in the photooxidation of proteins (e.g., dopa decarboxylase. In addition, proflavine can inhibit a number of enzymes directly. For xample scriflavine, a mixture of proflavine with 3,6-diamino-10-methylacridinium chloride, inhibits protein kinase C. Target(s): L-amino-acid oxidase; carbonic anhydrase, or carbonate dehydratase, Ki ≈ 10 mM; chymotrypsin; DNA (cytosine-5-)-methyltransferase; DNA-directed RNA polymerase; dopa decarboxylase; F1Fo ATPase, or F1Fo ATP synthase, or H+-transporting two-sector ATPase; ficain, or ficin; glutamate decarboxylase; lysozyme; monoamine oxidase; NADH:cytochrome b5 reductase; nucleoside-triphosphatase, nuclear-envelope; papain; poly(ADP-ribose) glycohydrolase; polynucleotide adenylyltransferase, or poly(A) polymerase; pyruvate kinase; RNA-directed DNA polymerase; thrombin; tRNA adenylytltransferase; tRNA (guanine-N2-)-methyltransferase; tRNA cytidylyltransferase; tRNA methyltransferases; tRNA nucleotidyltransferase; and trypsin.
Purification Methods
Proclavine (3,6-diaminoacridine) [92-62-6] M 209.2, m 284-286o, pK 1 -2.7, pK 2 0 . 5 5 , pK 3 9.49. It crystallises from aqueous MeOH. The picrate crystallises from aqueous pyridine with m ~185o. [Beilstein 22 H 487, 22 I 649, 22 II 397, 22 III/IV 5487, 22/11 V 322.] For proflavin see 3,6-diaminoacridine hydrochloride.