Description
Chymostatin is a bioactive peptide of microbial origin that acts as a protease inhibitor with selectivity for chymotryptase-
like serine proteases. It potently inhibits chymotrypsin and chymase (K
i = 9.36 and 13.1 nM, respectively) while less effectively blocking the activity of cathepsins, papain, and leukocyte elastase. It is without effect on trypsin, thrombin, plasmin, pepsin, and kallikrein.
Uses
Chymostatin is a specific inhibitor of α-, β-, γ-, and δ-chymotrypsin.
Uses
Chymostatin has been used in a study that determined that molecular calculations are useful for evaluating the interactions between ligands, including inhibitors and homologous enzymes, in docking models. Chymostatin has also been used in a study to investigate the norovirus protease as an attractive target for antiviral drug development.
General Description
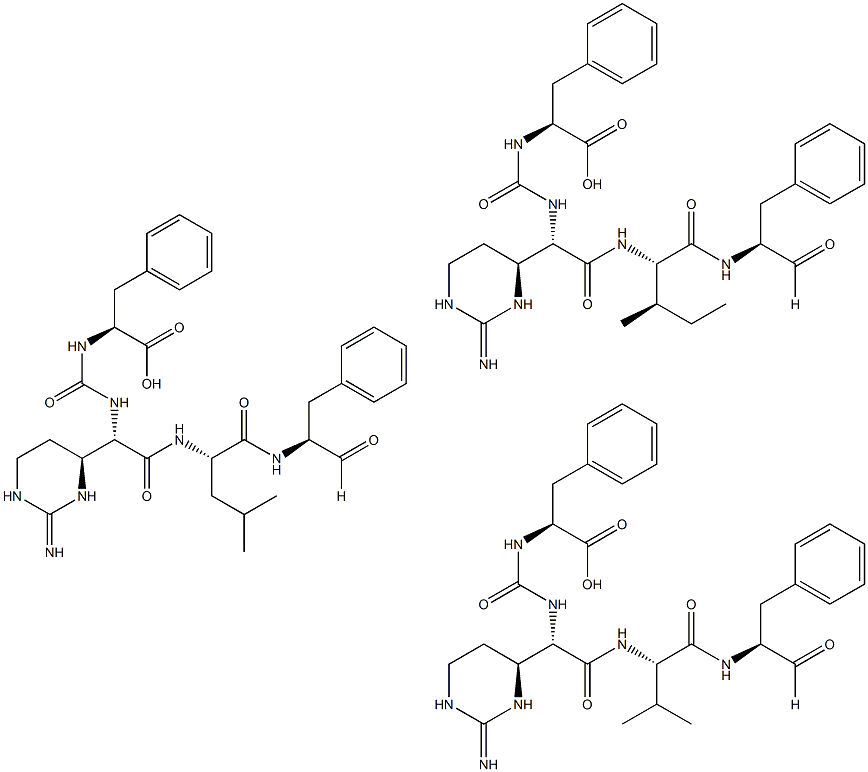
Chymostatin is a mixture of three components, A, B, and C. The component A being
N-[((S)-1-carboxy-2-phenylethyl)-carbamoyl]-α-[2-iminohexahydro-4(S)-pyrimidyl]-L-glycyl-L-leucyl-phenylalaninal. The other two components B and C differ in that the L-leucyl residue is substituted by L-valine and L-isoleucine, respectively.
Biochem/physiol Actions
Chymostatin is a strong inhibitor of many proteases, including chymotrypsin, papain, chymotrypsin-like serine proteinases, chymases, and lysosomal cysteine proteinases such as cathepsins A,B,C, B, H, and L. It weakly inhibits human leucocyte elastase. It is effective at a final concentration of 100 to 200 μg/ml (10 to 100 μM). Chymostatin is often included in protease inhibitor cocktails used with plant extracts.
in vitro
chymostatin was found to be a potent inhibitor of human leucocyte chymotrypsin-like protease. its affinity to the leucocyte protease was found to be much higher than its affinity to bovine pancreatic alpha-chymotrypsin. chymsotatin also showed a weak inhibitory effect on the activity of human leucocyte elastase. in addition, the preincubation of chymostatin with 35so2-4-labeled cartilage before the addition of the human chymotrypsin-like protease to the tissue could also inhibit 35so2-4 release [1].
in vivo
the effect of chymostatin on c57bl/6j-dy dystrophic mice was studied. the locomotor activity of normal mice markedly increased, attaining a plateau at 8 weeks of age. serum levels of creatine phosphokinase were much higher in dystrophic mice when compared with normal mice, and dystrophic mice also showed a reduced muscle protein content. when 3-week-old dystrophic mice received chymostatin at 1 mg/kg, the decrease in locomotor activity could be retarded, serum enzyme levels significantly decreased, and muscle protein content also obviously increased. in addition, the survival time of chymostatin-treated dystrophic mice was prolonged [2].
References
1) Umezawa?et al.?(1970),?Chymostatin, a new chymotrypsin inhibitor produced by actinomycetes; J. Antibiot. (Tokyo)?23?425
2) Johnson?et al.?(1998),?Inactivation of chymotrypsin and human skin chymase: kinetics of time-dependent inhibition in the presence of substrate; Biochim. Biophys. Acta?953?269
3) Stein and Strimpler (1987),?Slow-binding of chymotrypsin and cathepsin G by the peptide aldehyde chymostatin; Biochemistry?26?2611
4) Roszkowska-Chojecka?et al.?(2015),?Effects of chymostatin, a chymase inhibitor, on blood pressure, plasma and tissue angiotensin II, renal haemodynamics and renal excretion in two models of hypertension in the rat; Exp. Physiol.?100?1093