Uses
Synthesis of dyestuffs, explosives, celluloid
production
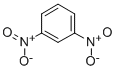
Definition
ChEBI: 1,2-dinitrobenzene is a dinitrobenzene.
General Description
One or more of the three isomeric (1,2- 1,3- and 1,4-) dinitrobenzenes, which are solids at room conditions, presumably in a non-aqueous solvent or carrier. Toxic by skin absorption. Exposure of the confined material to heat or shock may result in explosive decomposition. Produces toxic oxides of nitrogen during combustion.
Reactivity Profile
dinitrobenzene may react vigorously with oxidizing materials. Reaction with nitric acid (nitration) leads to a mixture of trinitrobenzenes possessing high-explosive properties [Urbanski, 1967, vol. 3, p. 290]. If heat and reaction conditions of the nitration are not controlled, detonation comparable to that of TNT may occur [Anon., J. R. Inst. Chem., 1960, 84, p. 451]. A mixture of 1,3-dinitrobenzene with tetranitromethane can be highly explosive [Urbanski, 1964, vol. 1, 592]. 1,2-dinitrobenzene is a severe explosion hazard when shocked or exposed to heat or flame.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
Suspected carcinogen.
A poison. When heated to decomposition it
emits toxic fumes of NOx. See also oDINITROBENZENE.
Carcinogenicity
In vitro studies show that m-DNB is mutagenic
in Salmonella typhimurium.
The 2003 ACGIH threshold limit valuetime-
weighted average (TLV-TWA) for all
isomers of dinitrobenzene is 0.15 ppm (1.0mg/
m3) with a notation for skin absorption.