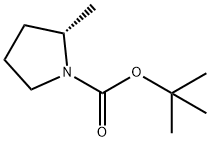
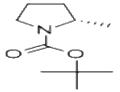
Synthesis
Tert-butyl 2R-methanesulfonyloxymethylpyrrolidine-1-carboxylate (1.6 g, 5.73 mmol) was used as a raw material, and lithium triethylborohydride (1 M solution in THF, 17 mL, 17 mmol) was slowly added to its solution in 10 mL of tetrahydrofuran at 0 °C. The reaction mixture was gradually warmed to room temperature and stirred continuously for 16 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate and water and washed sequentially with 0.1 N HCl and saturated saline. The organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated to give tert-butyl (S)-2-methylpyrrolidine-1-carboxylate as a colorless transparent oil (0.930 g, 88% yield). Its NMR hydrogen spectrum (400 MHz, CDCl3) data were as follows: δ 3.83 (m, 1H), 3.38 (m, 2H), 1.92 (m, 3H), 1.58 (m, 1H), 1.48 (s, 9H), 1.2 (d, J = 8 Hz, 3H).
References
[1] Patent: WO2006/19833, 2006, A1. Location in patent: Page/Page column 61
[2] Tetrahedron Letters, 1995, vol. 36, # 8, p. 1223 - 1226
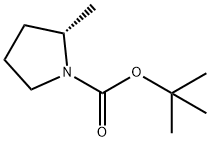



![tert-butyl (2R)-2-[(methanesulfonyloxy)methyl]pyrrolidine-1-carboxylate](/CAS/20200331/GIF/132482-10-1.gif)