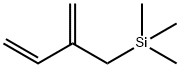
Physical properties
bp 69–70 °C/80 mmHg.
Uses
2-Trimethylsilylmethyl-1,3-butadiene is an isoprenylation reagent; Diels–Alder diene; reactant in Formation of π-Allylic Complex Followed by Acyldemetalation.
Preparation
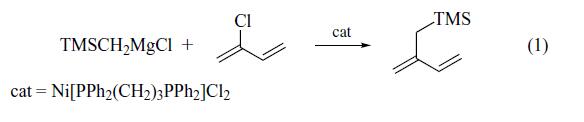
2-Trimethylsilylmethyl-1,3-butadiene is prepared most conveniently by the
coupling reaction of the Grignard reagent prepared from
(chloromethyl)trimethylsilane with 2-chloro-1,3-butadiene
(chloroprene) in the presence of a catalytic amount of Ni[Ph2P-
(CH2)3PPh2]Cl2 (91% yield) (eq 1). Alternatively, it can be
prepared by the reaction of the same Grignard reagent with allenylmethyl
phosphate in 57¨C73% yield (eq 2). Direct metalation
of isoprene followed by the reaction with chlorotrimethylsilane
gives 2-trimethylsilylmethyl-1,3-butadiene in low
yield. Thermal isomerization of 1-trimethylsilylmethylcyclobutene
to 2-trimethylsilylmethyl-1,3-butadiene has also
been reported.
storage
Silane, trimethyl(2-methylene-3-buten-1-yl)- can be stored in a glass bottle under nitrogen.
Purification Methods
Silane, trimethyl(2-methylene-3-buten-1-yl)- is purified by distillation.