Description
MC-MMAF is an antibody that binds to the epidermal growth factor receptor (EGFR) on tumor cells and inhibits the proliferation of tumor cells by blocking the binding of epidermal growth factor. MC-MMAF has been shown to be an effective treatment for a variety of cancers, including breast cancer, lung cancer, head and neck cancer, and colorectal cancer. It is also being studied as a potential treatment for autoimmune diseases such as rheumatoid arthritis and psoriasis. MC-MMAF has been shown to improve prognosis in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2.
Uses
Mafodotin is an anticancer immunoconjugate for the diagnosis and treatment of cancer or B Cell proliferative diseases.
Definition
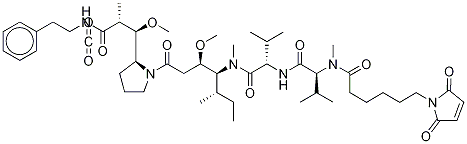
Maleimidocaproyl-monomethyl auristatin F, also known as MC-MMAF, is a precursor of antibody drug conjugate. MMAF and monomethyl auristatin E (MMAE) are derivatives of dolastatin 10. Both of which possess an N-terminal secondary amine (rather than the tertiary amine present in dolastatin 10), allowing for straightforward linker attachment. MMAF is less able to cross cell membranes than MMAE due to its C-terminal carboxylic acid group, but MMAF is also more hydrophilic, has a lesser tendency to aggregate and shows lower systemic toxicity than MMAE[1].
Application
SGD 1269 is a potent tubulin inhibitor and is a toxin payload in antibody drug conjugate. It is a useful agent for make antibody drug conjugate (ADC) for targeted drug delivery.
Biological Activity
McMMAF is a protective group (maleimidocaproyl)-conjugated MMAF, which is a potent tubulin polymerization inhibitor. McMMAF can be used as a drug-linker for?antibody-drug conjugates (ADC). McMMAF ADCs are uncleavable, and must be internalized and degraded within a cell, releasing cysteine-McMMAF as the active drug.
in vivo
Dose response of anti-tumor activity, characterized in the Bjab-Luc xenograft model, yielded an ED50 of ~1 mg/kg for MC-MMAF. MCC-DM1 was about 2-fold less potent compared to MC-MMAF in this model. Conjugation with DM1 or MMAF appeared to have minimal impact on the PK of anti-CD22 when the PK of naked anti-CD22 was compared with the total antibody PK profile in all three species. Pharmacokinetic parameters of both total antibody and ADCs were generally dose proportional at the dose range studied. Free DM1 in plasma increased with dose, while free MMAF was not detected[2].
References
[1] Baah S, et al. Antibody–Drug Conjugates—A Tutorial Review. Molecules, 2021; 26: 2943.
[2] Xie D, et al. Non-Clinical Pharmacology of Anti-CD22 Antibody Drug Conjugates. Blood, 2007; 110: 2363.