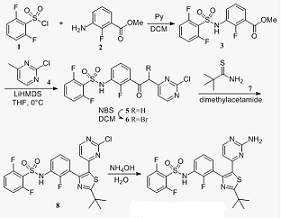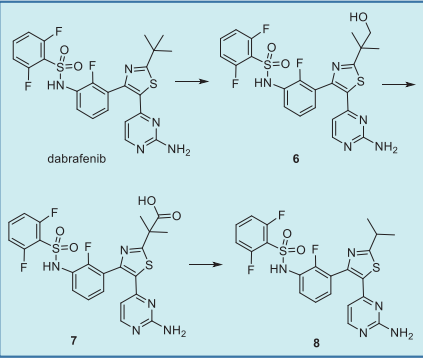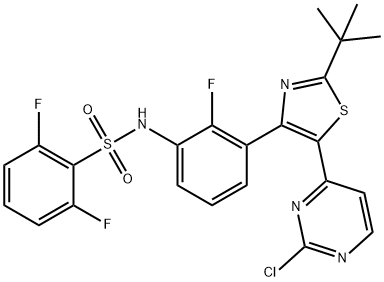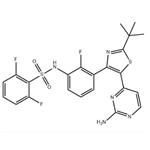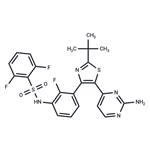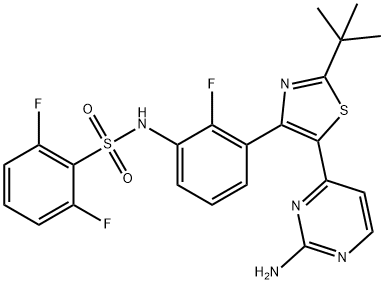
Dabrafenib
- Product NameDabrafenib
- CAS1195765-45-7
- CBNumberCB32604230
- MFC23H20F3N5O2S2
- MW519.56
- EINECS689-166-9
- MDL NumberMFCD17215684
- MOL File1195765-45-7.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 214-216oC |
| Boiling point | 653.7±65.0 °C(Predicted) |
| Density | 1.443 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 30 mg/ml with warming), or in Ethanol (up to 1 mg/ml with warming). |
| form | White solid. |
| pka | 6.62±0.10(Predicted) |
| color | Off-white |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| InChIKey | BFSMGDJOXZAERB-UHFFFAOYSA-N |
| SMILES | C1(S(NC2=CC=CC(C3=C(C4C=CN=C(N)N=4)SC(C(C)(C)C)=N3)=C2F)(=O)=O)=C(F)C=CC=C1F |
| FDA UNII | QGP4HA4G1B |
| ATC code | L01EC02 |
Safety
| Symbol(GHS) |
 
|
| Signal word | Warning |
| Hazard statements | H361-H411-H400 |
| Precautionary statements | P273-P391-P501-P201-P202-P281-P308+P313-P405-P501 |
| HS Code | 29350090 |