Description
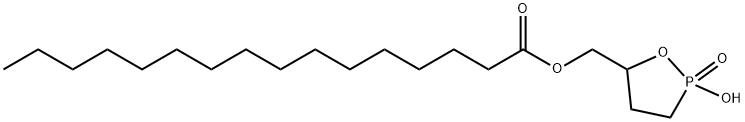
Cyclic phosphatidic acids (cPAs) are naturally occurring analogs of lysophosphatidic acid (LPA) in which the
sn-
2 hydroxy group forms a 5-
membered ring with the
sn-
3 phosphate. Carba-
derivatives of cPA (ccPA) are modified at the
sn-
2 (2-
ccPA) or
sn-
3 (3-
ccPA) linkage, preventing the opening of cPA to produce LPA. Palmitoyl 3-
ccPA is a cyclic LPA analog that contains the 16:0 fatty acid, palmitate, at the
sn-
1 position of the glycerol backbone. At 25 μM, it inhibits the transcellular migration of MM1 cells across mesothelial cell monolayers in response to fetal bovine serum (81.9%) or LPA (98.9%) without affecting proliferation. 3-
ccPA 16:0, at 0.1-
25 μM, significantly inhibits autotaxin, an enzyme that is important in cancer cell survival, growth, migration, invasion and metastasis.