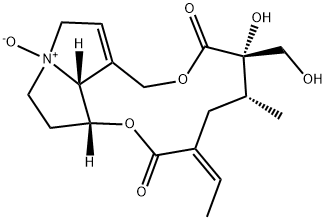
isatidine
- Product Nameisatidine
- CAS15503-86-3
- CBNumberCB31306005
- MFC18H25NO7
- MW367.39
- MDL NumberMFCD00074872
- MOL File15503-86-3.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 145°C |
| Boiling point | 497.95°C (rough estimate) |
| Density | 1.3204 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| pka | 11.96±0.40(Predicted) |
| FDA UNII | 51819GRV4U |
| IARC | 3 (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Isatidine (15503-86-3) |
Safety
| Symbol(GHS) |
 
|
| Signal word | Danger |
| Hazard statements | H373-H300 |
| Precautionary statements | P260-P314-P501-P264-P270-P301+P310-P321-P330-P405-P501 |
| RIDADR | 1544 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| Hazardous Substances Data | 15503-86-3(Hazardous Substances Data) |