Reactivity Profile
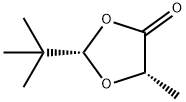
1,3-Dioxolan-4-one, 2-(1,1-dimethylethyl)-5-methyl-, (2S,5S)- is a cyclic acetals from (R)- and (S)-lactic acid and pivaldehyde; reagents for the preparation of enantiopure α- hydroxy-α-methyl carboxylic acids by alkylation of the corresponding lithium enolate with alkyl, allyl, and benzyl halides, by hydroxyalkylation with aldehydes and ketones, and by Michael addition to nitroalkenes; precursor to the 5-bromo derivative used for radical reactions; precursor to 2-t-butyl-5-methylene-1,3-dioxolan-4-one; an acceptor for radical additions; and an ene component for Diels-Alder reactions leading to cyclic, heterocyclic, and bicyclic α-hydroxy carboxylic acids.
Synthesis
On a 0.5 mol scale reagent 1,3-Dioxolan-4-one, 2-(1,1-dimethylethyl)-5-methyl-, (2S,5S)- is prepared by condensation of Pivalaldehyde and (S)-lactic acid under acid catalysis in pentane, with azeotropic removal of the water formed. The crude product is distilled in vacuo to give 93% of a 4:1 cis/trans mixture. Two recrystallizations from pentane/ether at -75 °C furnish 60% 1,3-Dioxolan-4-one, 2-(1,1-dimethylethyl)-5-methyl-, (2S,5S)- (cis/trans = 96:4).