Description
1,3,5-hexatriene is the simplest conjugated triene, and its structure predicts two geometrical isomers, the cis form being the open chain analog of benzene.
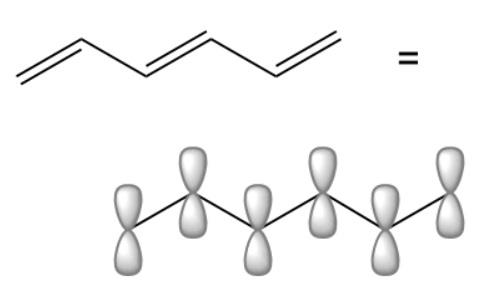
With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure. There are six adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these six atoms will result in six pi molecular orbitals: ψ1, ψ2, ψ3, ψ4*, ψ5*, and ψ6* (also referred to as π1, π2, π3, π4*, π5*and π6*).
Preparation
1,3,5-Hexatriene has been prepared by pyrolysis of s-divinylglycol diformate, condensation of allyl chloride with sodamide, phthalic anhydride dehydration of 1,5-hexadiene-3-ol and debromination of 3,4-dibromohexa-diene-1,5 with zinc. The product in method contained cyclohexadiene, and the purification of the substance seems to have been incomplete in all cases.




