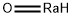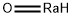Physical properties
Radium Oxide can be prepared by heating radium
metal in air to give a mixture of white radium oxide,
RaO, and radium nitride, Ra3N2. The superoxide RaO2
is also likely to form in this reaction.
2Ra(solid)+ O2(gas) → 2RaO(solid)
Ra(solid)+ O2(gas) → RaO2(solid)
3Ra(solid)+N2(gas) → Ra3N2(solid)
Radium reacts very readily with water to form
radium hydroxide, Ba(OH)2 and hydrogen gas (H2). The reaction is expected to be more violent than that of
barium:
Ra(solid)+ 2H2O(gas)→ Ra(OH)2(aq)+H2(gas)