Uses
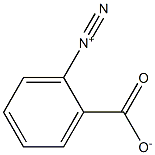
Benzenediazonium-2-carboxylate is used as a benzyne precursor.
Preparation
Benzenediazonium-2-carboxylate is obtained by diazotization of anthranilic acid; the most useful diazotization agent is Isopentyl Nitrite (caution, heart stimulant!).
Definition
ChEBI: The aromatic diazonium ion that is diazotised 2-aminobenzoic acid.
Precautions
Benzenediazonium-2-carboxylate can be generated in situ,2 handled as a suspension of the inner salt or the hydrochloride in ethers or halogenated hydrocarbons, or isolated as a dry solid. Solid benzenediazonium 2-carboxylate decomposes explosively on being heated or scraped against a hard surface. The use of the wet compound is safer, but explosions have been reported during experiments using a slurry of the inner salt or the hydrochloride. The safest procedure is generation in situ by aprotic diazotization, but yields are lower. Preparation and handling should be carried out with good shielding.