Synthesis
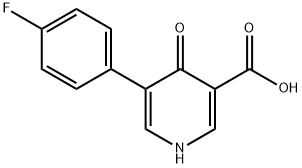
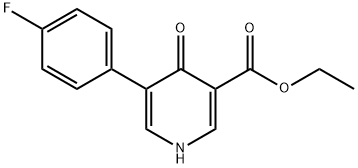
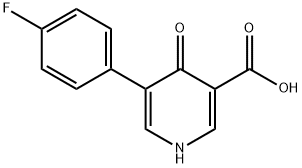
Example A8: Example A7 (0.750 g, 2.87 mmol) was suspended in 3 M NaOH aqueous solution (7.5 mL, 22.50 mmol) and heated to reflux at 100 °C for 1.5 hrs. Upon completion of the reaction, the mixture was cooled to room temperature and slowly acidified to pH 2 with 3 M aqueous HCl. The precipitated white solid was collected by vacuum filtration and washed with deionized water and dried to afford 5-(4-fluorophenyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid (669 mg, 100% yield). The product was analyzed by mass spectrometry (ESI), m/z: 234.1 ([M + H]+).
References
[1] Patent: WO2014/145004, 2014, A1. Location in patent: Paragraph 0111
[2] Journal of Medicinal Chemistry, 2008, vol. 51, # 17, p. 5330 - 5341
[3] Patent: WO2009/94417, 2009, A1. Location in patent: Page/Page column 28; 35-36
[4] Patent: WO2014/713, 2014, A1. Location in patent: Page/Page column 38