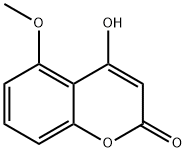
Synthesis
4-Hydroxy-5-methoxy-2H-1-benzopyran-2-one is obtaned by 2-Hydroxy-5-methoxy-acetophenone with sodium hydride in diethyl carbonate.2-Hydroxy-5-methoxy-acetophenone (1.50 g, 9.03 mmol) in diethyl carbonate (8.3
ml) was added dropwise into a suspension of sodium hydride (60% dispersion in
mineral oil, 1.81 g, 45.2 mmol) in diethyl carbonate (8.3 ml) and heated under
reflux at 100 ?C for 3 hours. The resultant mixture was left to cool to 0 ?C in an ice
bath and quenched by dropwise addition of water until effervescence ceased. The
aqueous layer was washed with diethyl ether (3 × 16 ml) then acidified using
concentrated hydrochloric acid to pH 1. The precipitate was filtered and washed
with water, followed by petroleum ether and left to dry overnight at 90 ?C to give
the title compound as a colourless solid (902 mg, 52%): mp: 150 – 153 ?C (Lit.: 155
?C); vmax/cm-1 3207bw (O-H), 1727s (C=O), 1655m (C=C), 1606s (aro. ring
C=C); δH (300 MHz; DMSO-d6) 11.34 (1H, br. s., OH), 7.57 (1H, t, J 8.4, C(7)H),
6.95 (2H, d, J 8.4, C(6)H and C(8)H), 5.51 (1H, s, C(3)H), 3.90 (1H, s, OCH3); δC
(75 MHz; DMSO-d6) 167.2 (Ar-C), 161.4 (Ar-C), 157.3 (Ar-C), 155.2 (Ar-C),
133.0 (Ar-CH), 109.2 (Ar-CH), 106.8 (Ar-CH), 105.0 (Ar-C), 90.8 (C(3)H), 56.5
(OCH3); m/z (-ES) 191 (100%, [M-H]-).